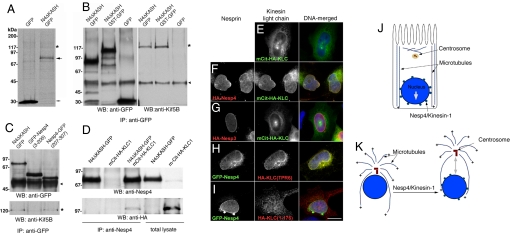
Fig. 4.
Nesp4 interacts with kinesin-I. (A) Anti-GFP IP analysis of 35S-Met/Cys-labeled HEK293 cells expressing either Nesp4ΔKASH–GFP (large arrow) or GFP alone (small arrow). A faint ≈120-kDa band (*) present only in the Nesp4ΔKASH–GFP IPs was found, by mass spectrometry, to contain Kif5B, the kinesin-1 heavy chain. (B) Unlabeled HeLa cells expressing Nesp4ΔKASH–GFP, Nesp4ΔKASH–GST–GFP, or GFP were processed for IP using anti-GFP. IPs were analyzed by Western blot using either anti-GFP or anti-Kif5B. Kif5B (*) was clearly evident in IPs containing either Nesp4ΔKASH–GFP or Nesp4ΔKASH–GST–GFP. Ig heavy chains are indicated (arrowhead). (C) Similar analysis of HeLa cells expressing Nesp4ΔKASH–GFP, GFP–Nesp4(3–206), and Nesp4–GFP(207–307). Kinesin-1 coimmunoprecipitates only with Nesp4ΔKASH–GFP and Nesp4–GFP(207–307). (D) HeLa cells were transfected with various combinations of Nesp4ΔKASH–GFP and with KLC1 fused to a fluorescent protein and HA-tagged mCit-HA-KLC1. IP of Nesp4 followed by Western blot analysis using both anti-Nesp4 and anti-HA antibodies reveals association between Nesp4 and mCit–HA–KLC1. Immunofluorescence microscopy of HeLa cells expressing various combinations of nesprins and KLC1. (E) mCit–HA–KLC1 alone localizes to the cytoplasm. (F) HA–Nesp4 recruits mCit–HA–KLC1 to the NE. (G) Nesp3 has no effect on mCit–HA–KLC1. (H) An HA-tagged version of the KLC1 TPR domain [HA-KLC(TPR6)] is recruited to the NE by GFP–Nesp4. (I) The distribution of HA–KLC(1–176), which lacks the TPR domain, is unaffected by GFP–Nesp4. In the merged images, DNA, revealed by staining with Hoechst dye, is shown in blue. (Bar: 10 μm.) (J) In polarized epithelial cells microtubules are noncentrosomal. The bulk are arranged in lateral bundles with their plus (+) ends oriented toward the basal membrane. Kinesin-1 on the NE should drive the nucleus toward the base of the cell. (K) Nesp4-mediated recruitment of kinesin-1 to the NE in fibroblasts should induce separation of the nucleus and centrosome.