Abstract
Neurons containing melanin-concentrating hormone (MCH) are codistributed with neurons containing orexin (Orx or hypocretin) in the lateral hypothalamus, a peptide and region known to be critical for maintaining wakefulness. Evidence from knockout and c-Fos studies suggests, however, that the MCH neurons might play a different role than Orx neurons in regulating activity and sleep–wake states. To examine this possibility, neurons were recorded across natural sleep–wake states in head-fixed rats and labeled by using the juxtacellular technique for subsequent immunohistochemical identification. Neurons identified as MCH+ did not fire during wake (W); they fired selectively during sleep, occasionally during slow wave sleep (SWS) and maximally during paradoxical sleep (PS). As W-Off/Sleep-On, the MCH neurons discharged in a reciprocal manner to the W-On/Sleep-Off Orx neurons and could accordingly play a complementary role to Orx neurons in sleep–wake state regulation and contribute to the pathophysiology of certain sleep disorders, such as narcolepsy with cataplexy.
Keywords: hypocretin, narcolepsy, paradoxical sleep, REM sleep, slow wave sleep
Since early neuropathological and lesion studies, the lateral hypothalamus (LH) has been considered to be critical for the maintenance of the waking state (1). More recently, neurons that contain the peptide orexin (Orx, or hypocretin) were localized to the LH (2), and Orx was found to be a necessary neuromodulator for the maintenance of waking, because, in its absence, narcolepsy with cataplexy occurs in animals and humans (3–6). Codistributed with neurons containing Orx are neurons containing the peptide melanin-concentrating hormone (MCH) (7). Although MCH was thought to act like Orx as an orexigenic peptide in food consumption, it appeared to act in an opposite manner to Orx on activity and energy consumption. Whereas Orx knockout mice were narcoleptic, obese, and hypometabolic (3, 8), MCH knockout mice were hyperactive, lean, and hypermetabolic (9). It thus appeared that MCH might influence sleep–wake states in a different manner than Orx. Several studies explored this possibility by examining c-Fos expression in relation to sleep–wake states. Whereas Orx neurons expressed c-Fos after continuous waking maintained by total sleep deprivation, MCH neurons expressed c-Fos after recovery from sleep deprivation, suggesting that they could be active during slow wave sleep (SWS) and/or rapid eye movement (REM), also called paradoxical sleep (PS) (10). In another study, MCH neurons expressed c-Fos after recovery from PS deprivation and thus particularly in association with PS (11). However, other studies failed to see expression of c-Fos in MCH neurons after enhanced natural SWS or carbachol-induced PS (12). However, c-Fos expression is not a simple function of neural discharge or increment in discharge but depends on the temporal pattern of action potentials and also receptor activation (13, 14). Only by recording the activity of specific neurons in association with polygraphic and behavioral correlates can their discharge profile and potential role in sleep–wake states be established. Because, MCH, like Orx, neurons represent <10% of the cell population in the LH, their specific characterization necessitates their immunohistochemical identification, which is possible by using juxtacellular labeling of recorded cells in naturally sleeping–waking, head-fixed rats. With this procedure, we established that Orx neurons fire maximally during waking and virtually cease firing during sleep, including SWS and PS (15). In the present study, we used this approach to determine the discharge profile of identified MCH neurons in relation to natural sleep–wake states and their electroencephalographic (EEG) and electromyographic (EMG) correlates.
Results
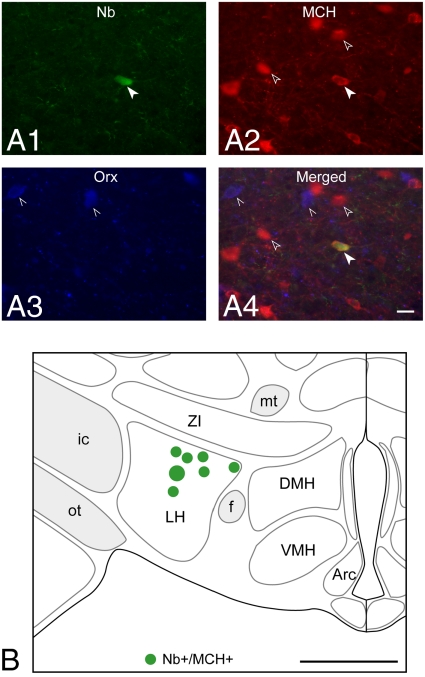
Neurons recorded across the full sleep–wake cycle were labeled by the juxtacellular technique with Neurobiotin (Nb), only one cell per side per rat, before perfusion and fixation of the brain. After staining of sections for Nb, sections containing Nb-labeled cells were processed for dual-immunostaining of MCH and Orx (Fig. 1A). Nb+/MCH+ neurons were located in the LH in the same region and often in close proximity to Orx neurons (LH, Fig. 1 A and B).
Fig. 1.
Recorded, Nb-labeled, MCH-immunopositive neurons were located near the Orx neurons in the lateral hypothalamus. (A) Fluorescence image of Nb-labeled (green, Cy2, filled arrowhead in A1) neuron (c134u01), which was immunopositive for MCH (red, Cy3, arrowheads in A2) and located near codistributed Orx+ neurons (blue, Cy5, pointers in A3 and in merged image in A4). (Scale bar, 25 μm.) (B) Location of recorded, Nb+/MCH+ neurons (n = 7) on a coronal atlas section (≈ −2.8 mm from bregma or 6.6 mm anterior to interaural zero) through the lateral hypothalamus. The cell shown in A is represented as the largest symbol in B. (Scale bar, 1 mm.) Arc, arcuate nucleus; DMH, dorsomedial hypothalamic nucleus; f, fornix; ic, internal capsule; LH, lateral hypothalamus; mt, mammillo-thalamic tract; ot, optic tract; VMH, ventromedial hypothalamic nucleus; ZI, zona incerta.
In the search for MCH+ neurons, a total of 96 units were successfully recorded across the full sleep–wake cycle, labeled with Nb, located in the LH, and judged unequivocally immunopositive (+) or immunonegative (−) for MCH and Orx. Cells with all profiles of discharge were sampled during the search, thus allowing a clear assessment of which cell types were not MCH+ along with those that finally proved to be MCH+ (Table 1). Cells that did not manifest a significant change in their firing rate across sleep–wake states, called “wsp-eq” (wake, SWS, PS-equivalent), were not MCH+ (n = 10). Cells that fired at high rates during both active wake (aW) and PS, called “WP-max,” and thus in positive association with cortical activation and EEG gamma activity, were not MCH+ (n = 17). Cells that fired maximally during aW and minimally during sleep, as “W-max,” and thus in positive association with EMG amplitude, were not MCH+, but included 6 (of 19) cells that were Orx+, as reported (15) (Table 1). Cells that fired maximally during SWS, as “S-max,” and thus in positive association with delta EEG activity, were not MCH+ (n = 9). Finally, Nb-labeled cells that fired maximally during PS, as “P-max,” and thus in negative association with EMG activity, included 7 (of 41) cells that were MCH+.
Table 1.
Number of Nb-labeled MCH+, Orx +, or MCH−/Orx− neurons with different sleep–wake discharge profiles
| Sleep–wake discharge profile | Total | Nb+/MCH−/Orx− | Nb+/Orx+* | Nb+/MCH+ |
|---|---|---|---|---|
| wsp-eq | 10 | 10 | 0 | 0 |
| WP-max | 17 | 17 | 0 | 0 |
| W-max | 19 | 13 | 6 | 0 |
| S-max | 9 | 9 | 0 | 0 |
| P-max | 41 | 34 | 0 | 7 |
| Total | 96 | 83 | 6 | 7 |
By statistical analysis of their mean firing rate per second during active wake (W), SWS (S), or paradoxical sleep (P) (one-way ANOVA), units were distinguished as having equivalent firing rates across states (wsp-eq) or significantly different firing rates across states, in which case they were further classified according to the state or states during which their rate was maximal (by Bonferroni correction for multiple paired comparisons).
*Ref. 15.
It should also be mentioned that, during this long search, manipulations of food or sucrose intake were attempted in some animals according to results suggesting that MCH neurons would become active either after reduced food intake and negative energy balance (16) or conversely after increased glucose levels and positive energy balance (17). Although one MCH+ cell was found under the first condition (of 19) and one under the second condition (of 6), the probability of finding MCH+ cells was low under both conditions and the profile of the MCH+ cells was the same under both conditions. Indeed, after discovering that only cells that discharged maximally during PS under either condition were MCH+, the search for MCH+ cells was subsequently pursued under normal feeding conditions and focused particularly on cells discharging during sleep and maximally during PS. Despite the subsequent preferential selection of P-max cells, the total number of identified MCH+ cells represented ≈7% of all of the Nb-labeled neurons (n = 96) in our sample (Table 1), which is similar to the proportion of MCH+ cells in the total cell population within the LH (10).
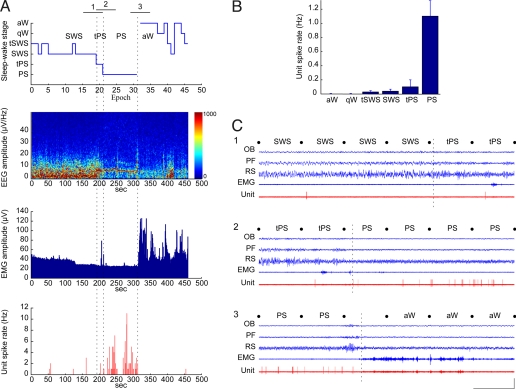
The MCH+ neurons did not fire during active (aW) or quiet W (qW). They fired very occasionally during the transition into SWS (tSWS) and SWS. They emitted a few spikes in the transition to PS (tPS) and then discharged at their maximum rate during PS (Fig. 2). The average discharge rate of MCH+ neurons during PS was quite low, 1.1 ± 0.26 Hz (mean ± SEM for 6 cells having >1-min epochs per state), whereas their average instantaneous firing frequency was much higher, 21.73 ± 7.86 Hz, reflecting a phasic pattern of firing [supporting information (SI) Fig. S1 A and B]. The average duration of their individual spikes was 2.06 ± 0.23 ms (n = 7) (Fig. S1C). During tSWS, SWS, or tPS, they tended to fire in doublets (Fig. 2C1 and Movie S1). During PS, they tended to fire phasically also in doublets or groups of spikes and often so during periods of rapid movements or twitches of the whiskers and muzzle (Fig. 2C2 and Movie S2). Yet, they also discharged during quiet periods of PS. During the transition from PS to aW, they ceased firing completely on arousal and the return of neck muscle tone to remain silent during both aW and qW (Fig. 2C3 and Movie S3). Across the sleep–wake cycle, the MCH neurons thus discharged during periods of low muscle tone (Fig. 2 A and C), and their discharge rate was negatively correlated with EMG amplitude (r = −0.42, in 4 rats having good EMG recordings).
Fig. 2.
Nb+/MCH+ neurons discharged only during sleep. (A) Data from Nb+/MCH+ unit (c134u01, Fig. 1) showing the sleep–wake stage scored per 10-s epoch, together with simultaneous EEG amplitude (μV/Hz with frequency on y axis and amplitude scaled according to color), EMG amplitude (μV), and unit spike rate (Hz) per second of the recording session. Note that the unit fired during SWS or tSWS, tPS, and PS when EEG showed prominent slow (<5 Hz) or theta (≈6–8 Hz) activity and EMG amplitude was relatively low. Vertical dashed lines indicate precise moments of transition between SWS and tPS, tPS and PS, and PS and aW here and within corresponding segments 1, 2, and 3 in C. (B) Mean spike rate (Hz) of the Nb+/MCH+ unit per sleep–wake stage. Note that it did not fire during aW or qW, fired very little during tSWS, SWS, and tPS, and fired maximally during PS. (C) Polygraphic records (from 1-min periods indicated in A) of the unit discharge along with EEG and EMG activity during passages between states: (1) from SWS to tPS, (2) from tPS to PS, and (3) from PS to aW. Note that the unit fired only very occasionally with 1 or 2 spikes during periods of SWS and tPS (shown in C1). It discharged phasically in groups of spikes to reach its highest rates during PS (shown in C2). It ceased firing on arousal (marked by dashed line indicating transition during uncategorized epoch) and during the subsequent aW period (shown in C3). Movies S1–S3 are also shown of 30-s segments of continuous SWS (1), PS (2), and aW (3) taken before (1) or after (2 and 3) the transitional periods shown in C. Calibrations: horizontal, 10 s; vertical, 1 mV (EEG, EMG), 2 mV (unit). OB, olfactory bulb; PF, prefrontal cortex; RS, retrosplenial cortex.
The MCH+ neurons did not comprise all of the Nb-labeled P-max neurons recorded in the LH and in fact represented only 17% of them (Table 1). The Nb+/MCH+ neurons differed on average from the Nb+/MCH−, P-max neurons, which had a significantly higher mean discharge rate (7.14 ± 1.02 Hz; P < 0.05 according to t test) and insignificantly higher mean instantaneous firing frequency (50.88 ± 8.79 Hz, P = 0.18) during PS along with a significantly narrower mean spike width (1.51 ± 0.06 ms; P < 0.01). However, on each of these variables, the 2 groups overlapped considerably such that unequivocal distinction of the MCH+ from the MCH− P-max cells proved impossible with use of any 1 or all 3 of these parameters (Fig. S2) and only possible by immunohistochemical staining of the Nb-labeled cells.
Interestingly, MCH+ neurons showed a reciprocal pattern of discharge to that of Orx+ neurons, which were previously identified by using juxtacellular labeling as W-max cells (15) (Fig. 3). Whereas the MCH+ neurons were silent during aW and qW and were most active during PS, the Orx neurons were most active during aW and virtually silent during PS, except during the last several seconds preceding waking. Neither MCH nor Orx neurons discharged in direct association with EEG gamma or delta activity. However, MCH neurons discharged in negative association with EMG amplitude, whereas Orx neurons discharged in positive association with EMG amplitude, reflecting postural muscle tone. Concerning other discharge properties, the MCH+ neurons did not differ significantly from Orx+ neurons, which had a relatively low average maximal discharge rate (3.17 ± 0.79 Hz) and instantaneous firing frequency (12.19 ± 2.9 Hz) and relatively long spike duration (2.02 ± 0.07 ms; P > 0.05, according to t tests for 6 or 7 MCH+ and 6 Orx+ neurons) (Fig. S3).
Fig. 3.
MCH neurons discharged in a reciprocal manner to Orx neurons across sleep–wake states. (A) The mean spike rate (Hz) per stage of Nb+/Orx+ units (n = 6; from ref. 15) varies in a reciprocal manner to that of Nb+/MCH+ units (n = 6; this study). (B) The reciprocal firing profiles were not correlated with EEG gamma or delta activity but were correlated in an inverse manner with EMG amplitude, positively for the wake-active Orx neurons and negatively for the MCH sleep-active neurons. EEG and EMG plotted as mean normalized amplitude from the same recordings as units.
Discussion
Applying juxtacellular recording and labeling of neurons in head-fixed rats, the present study reveals the discharge profile of identified MCH neurons across the sleep–wake cycle. Remarkably, MCH neurons did not fire during waking, fired occasionally during SWS, and fired maximally during PS. As Wake-Off/Sleep-On cells, their profile of discharge is reciprocal to that of Orx+ cells, which are Wake-On/Sleep-Off (15, 18).
The complete silence of the MCH neurons during both quiet and active wake was surprising because high activity during waking was reported for the majority of neurons in this wake-promoting region of the hypothalamus (19). The silence of MCH neurons during waking was also found in animals after food restriction or sucrose administration, 2 different conditions of energy balance that were proposed to stimulate activity in MCH neurons (16, 17). Actually, multiple in vivo studies indicate that MCH is activated by negative energy balance, whereas few indicate the inverse. Fasting, like leptin deficiency, results in increased expression of MCH mRNA (20) and cAMP response element (CRE) transcription in MCH neurons (16), particularly in the later phase of fasting when body fat is decreased (21). The up-regulation of MCH was reported to occur only during the resting phase (22), when rodents sleep the majority of the time. Because gene expression can be stimulated by neural discharge (14), increased MCH expression during sleep in response to fasting would be corroborated by the current results showing selective discharge of the MCH+ cells during sleep.
Previous evidence from studies examining expression of the immediate early gene, c-Fos, had indicated that MCH neurons would be more active during SWS and/or PS than during waking (10, 11). Yet, c-Fos together with other gene expression is not simply determined by the number or increment of action potentials (13, 14). It is likely for this reason that c-Fos is not evident in MCH neurons under baseline conditions in rats (10, 11) or under any conditions in cats (12), despite significant amounts of SWS and PS. That c-Fos expression in MCH neurons occurs with recovery from sleep deprivation could be due to a particular discharge pattern and/or receptor activation during enhanced SWS and PS. Whereas sleep recovery after total sleep deprivation for a short period produced c-Fos expression in a very small percentage of MCH neurons (10), sleep recovery after PS deprivation for a prolonged period produced c-Fos expression in a major proportion of MCH neurons (11). This difference could well be due to the more selective and enhanced increase in PS after PS deprivation in the latter study. Yet, it might also be due in part to changes in energy stores that follow prolonged total sleep or PS deprivation and include decreased body fat and serum leptin (23, 24), which stimulate gene expression in the MCH cells (above). Nonetheless, the c-Fos expression occurred in MCH cells only after 3 h of recovery sleep and not directly after the 72 h of PS deprivation (11). Interpreted in light of the present results, the immediate early genes, such as c-Fos, as well as other genes (above), are likely stimulated under appropriate conditions by the maximal discharge of the MCH neurons, which can only occur with the full sleep cycle culminating in PS.
Although they discharged occasionally during SWS, the MCH cells discharged fully only during the state of PS. They did not discharge fully during the transition into PS, nor continuously during PS. They accordingly did not appear to generate the state of PS but more likely to participate in certain of its components. It is perhaps for this reason that knockout of the MCH1 receptor did not result in decreases of PS, SWS, or total sleep (25). However, pharmacological antagonism of the MCH1 receptor did produce a dose-dependent decrease of PS and SWS in favor of waking (26). Moreover, knockout of the peptide, MCH resulted in significant decreases in SWS along with total sleep (27). Interestingly, MCH-deficient mice showed a greater decrease in sleep, including PS and SWS in response to fasting (27), indicating a particular role for MCH in the maintenance of sleep in relation to metabolic status.
The discharge of MCH neurons was not related to EEG activity but was negatively correlated with EMG activity, because it was highest during PS when muscle atonia occurred. Given that MCH neurons project into the brainstem and spinal cord where they may directly influence motor neurons (28), it is possible that they may serve to attenuate motor activity and muscle tone during PS. Interestingly, in MCH or MCH1 receptor knockout mice compared with wild type, motor activity is increased and most markedly after fasting (27, 29). Moreover, in these MCH-deficient mice, temperature, heart rate, and metabolic rate are increased along with energy expenditure (29). Conversely, infusion of MCH decreases temperature, heart rate, and metabolism (30, 31) and enhances SWS and PS (11). Given the profile of their discharge revealed here, it is thus possible that MCH neurons act to decrease temperature, heart rate, and metabolic rate along with motor activity by central and peripheral actions that enhance parasympathetic tone and attenuate sympathetic and somatomotor activity during sleep. MCH neurons could thus serve to conserve energy selectively during sleep. Indeed, during times of decreased food availability or fasting, which result in activation of MCH (above), natural decreases in body temperature and metabolic rate are selectively enhanced during sleep, whereas energy expenditure with food foraging is maintained during waking (32, 33).
It is strikingly revealed in this study that the MCH neurons discharge in a reciprocal manner to the Orx neurons across the sleep–wake cycle and in relation to muscle tone. Such reciprocal profiles of discharge could be due to the different intrinsic membrane properties of these cell groups, such that in vitro MCH neurons are spontaneously hyperpolarized and silent, whereas Orx neurons are depolarized and active (34). Or, they could be due to their different modulation by neurotransmitters of the arousal systems, MCH neurons being inhibited and Orx neurons being excited by NA, for example (35). These 2 systems, in turn, appear to have opposite effects on their target neurons, MCH exerting pre- and postsynaptic inhibitory effects, Orx exerting pre- and postsynaptic excitatory effects (36). MCH decreases motor activity, temperature, and metabolism while enhancing parasympathetic tone (above), whereas Orx increases motor activity, temperature, and metabolism while enhancing sympathetic tone (8, 37, 38). MCH induces sleep (11), whereas Orx evokes arousal (39). Blocking MCH receptors decreases sleep (26), whereas blocking Orx receptors increases sleep (40). The reciprocal profile of the MCH and Orx neurons thus reflects their complementary actions and roles in activity, temperature, metabolism, and integrated sleep–wake state generation.
The reciprocal profiles and roles of the Orx and MCH neurons could be significant in the manifestation of sleep disorders. It is possible that narcolepsy with cataplexy, which occurs with the loss of Orx neurons (4, 6), is provoked in part by the MCH neurons that remain intact in the narcoleptic patients (5). It is also interesting to note that, in depression, with which an elevated incidence of short-onset REM sleep periods and cataplexy occur (41), decreased Orx activity and enhanced MCH activity have been suggested to play a role (16, 42). Conversely, perhaps the recently documented loss of MCH neurons in Parkinson disease (43) could contribute to the absence of cataplexy and high incidence of “REM sleep without atonia” and “REM sleep behavior disorder” in this disease (44). Complementary roles of the Orx and MCH neurons in the hypothalamus could thus participate in the normal regulation of arousal, muscle tone, and sleep–wake states and disturbance to their reciprocal actions contribute to different symptoms involving sleep–wake states in neuropsychiatric disorders.
Methods
Recording and juxtacellular labeling of neurons was performed in adult male, Long–Evans rats, which previously had been operated on for implantation of EEG and EMG electrodes and a u-frame for head fixation and had been habituated to the head-fixed condition as described in ref. 15 (see SI Methods: Detailed Procedures). By using Nb-filled glass micropipettes, 1 unit recorded across aW, SWS, and PS was labeled per side per rat. After subsequent perfusion and fixation of the brain, Nb-labeled cells were located in frozen sections, which were, in turn, processed for dual-immunofluorescent staining for MCH and Orx.
Supplementary Material
Acknowledgments.
We thank Lynda Mainville for her technical assistance and Frederic Brischoux and Pablo Henny for their consultation. This work was supported by Canadian Institutes of Health Research Grant CIHR 82762 and National Institutes of Health Grant RO1 MH-60119–01A (to B.E.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811400106/DCSupplemental.
References
- 1.Jones BE. In: Principles and Practice of Sleep Medicine. Kryger MH, Roth T, Dement WC, editors. Philadelphia: Elsevier Saunders; 2005. pp. 136–153. [Google Scholar]
- 2.Peyron C, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chemelli RM, et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 4.Lin L, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 5.Thannickal TC, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peyron C, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 7.Broberger C, De Lecea L, Sutcliffe JG, Hokfelt T. Hypocretin/orexin- and melanin-concentrating hormone-expressing cells form distinct populations in the rodent lateral hypothalamus: Relationship to the neuropeptide Y and agouti gene-related protein systems. J Comp Neurol. 1998;402:460–474. [PubMed] [Google Scholar]
- 8.Hara J, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 9.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature. 1998;396:670–674. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 10.Modirrousta M, Mainville L, Jones BE. Orexin and MCH neurons express c-Fos differently after sleep deprivation vs. recovery and bear different adrenergic receptors. Eur J Neurosci. 2005;21:2807–2816. doi: 10.1111/j.1460-9568.2005.04104.x. [DOI] [PubMed] [Google Scholar]
- 11.Verret L, et al. A role of melanin-concentrating hormone producing neurons in the central regulation of paradoxical sleep. BMC Neurosci. 2003;4:19. doi: 10.1186/1471-2202-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torterolo P, Sampogna S, Morales FR, Chase MH. MCH-containing neurons in the hypothalamus of the cat: Searching for a role in the control of sleep and wakefulness. Brain Res. 2006;1119:101–114. doi: 10.1016/j.brainres.2006.08.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luckman SM, Dyball RE, Leng G. Induction of c-fos expression in hypothalamic magnocellular neurons requires synaptic activation and not simply increased spike activity. J Neurosci. 1994;14:4825–4830. doi: 10.1523/JNEUROSCI.14-08-04825.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fields RD, Eshete F, Stevens B, Itoh K. Action potential-dependent regulation of gene expression: Temporal specificity in Ca2+, cAMP-responsive element binding proteins, and mitogen-activated protein kinase signaling. J Neurosci. 1997;17:7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee MG, Hassani O, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georgescu D, et al. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burdakov D, Gerasimenko O, Verkhratsky A. Physiological changes in glucose differentially modulate the excitability of hypothalamic melanin-concentrating hormone and orexin neurons in situ. J Neurosci. 2005;25:2429–2433. doi: 10.1523/JNEUROSCI.4925-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alam MN, et al. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–631. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu D, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 21.Bertile F, Oudart H, Criscuolo F, Maho YL, Raclot T. Hypothalamic gene expression in long-term fasted rats: Relationship with body fat. Biochem Biophys Res Commun. 2003;303:1106–1113. doi: 10.1016/s0006-291x(03)00481-9. [DOI] [PubMed] [Google Scholar]
- 22.Harthoorn LF, Sane A, Nethe M, Van Heerikhuize JJ. Multi-transcriptional profiling of melanin-concentrating hormone and orexin-containing neurons. Cell Mol Neurobiol. 2005;25:1209–1223. doi: 10.1007/s10571-005-8184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koban M, Swinson KL. Chronic REM-sleep deprivation of rats elevates metabolic rate and increases UCP1 gene expression in brown adipose tissue. Am J Physiol. 2005;289:E68–E74. doi: 10.1152/ajpendo.00543.2004. [DOI] [PubMed] [Google Scholar]
- 24.Everson CA, Crowley WR. Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats. Am J Physiol. 2004;286:E1060–E1070. doi: 10.1152/ajpendo.00553.2003. [DOI] [PubMed] [Google Scholar]
- 25.Adamantidis A, et al. Sleep architecture of the melanin-concentrating hormone receptor 1-knockout mice. Eur J Neurosci. 2008;27:1793–1800. doi: 10.1111/j.1460-9568.2008.06129.x. [DOI] [PubMed] [Google Scholar]
- 26.Ahnaou A, et al. Blocking melanin-concentrating hormone MCH1 receptor affects rat sleep-wake architecture. Eur J Pharmacol. 2008;579:177–188. doi: 10.1016/j.ejphar.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 27.Willie JT, Sinton CM, Maratos-Flier E, Yanagisawa M. Abnormal response of melanin-concentrating hormone deficient mice to fasting: Hyperactivity and rapid eye movement sleep suppression. Neuroscience. 2008;156:819–829. doi: 10.1016/j.neuroscience.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito Y, Cheng M, Leslie FM, Civelli O. Expression of the melanin-concentrating hormone (MCH) receptor mRNA in the rat brain. J Comp Neurol. 2001;435:26–40. doi: 10.1002/cne.1191. [DOI] [PubMed] [Google Scholar]
- 29.Astrand A, et al. Mice lacking melanin-concentrating hormone receptor 1 demonstrate increased heart rate associated with altered autonomic activity. Am J Physiol. 2004;287:R749–R758. doi: 10.1152/ajpregu.00134.2004. [DOI] [PubMed] [Google Scholar]
- 30.Ito M, et al. Characterization of MCH-mediated obesity in mice. Am J Physiol. 2003;284:E940–E945. doi: 10.1152/ajpendo.00529.2002. [DOI] [PubMed] [Google Scholar]
- 31.Messina MM, Overton JM. Cardiovascular effects of melanin-concentrating hormone. Regul Pept. 2007;139:23–30. doi: 10.1016/j.regpep.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Wiersma P, Salomons HM, Verhulst S. Metabolic adjustments to increasing foraging costs of starlings in a closed economy. J Exp Biol. 2005;208:4099–4108. doi: 10.1242/jeb.01855. [DOI] [PubMed] [Google Scholar]
- 33.Rashotte ME, Pastukhov IF, Poliakov EL, Henderson RP. Vigilance states and body temperature during the circadian cycle in fed and fasted pigeons (Columba livia) Am J Physiol. 1998;275:R1690–R1702. doi: 10.1152/ajpregu.1998.275.5.R1690. [DOI] [PubMed] [Google Scholar]
- 34.Eggermann E, et al. The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J Neurosci. 2003;23:1557–1562. doi: 10.1523/JNEUROSCI.23-05-01557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bayer L, et al. Opposite effects of noradrenaline and acetylcholine upon hypocretin/orexin versus melanin concentrating hormone neurons in rat hypothalamic slices. Neuroscience. 2005;130:807–811. doi: 10.1016/j.neuroscience.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 36.Gao XB, van den Pol AN. Melanin concentrating hormone depresses synaptic activity of glutamate and GABA neurons from rat lateral hypothalamus. J Physiol. 2001;533:237–252. doi: 10.1111/j.1469-7793.2001.0237b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol. 1999;277:R1780–R1785. doi: 10.1152/ajpregu.1999.277.6.R1780. [DOI] [PubMed] [Google Scholar]
- 38.Monda M, Viggiano A, De Luca V. Paradoxical [correction of parodoxical] effect of orexin A: Hypophagia induced by hyperthermia. Brain Res. 2003;961:220–228. doi: 10.1016/s0006-8993(02)03953-7. [DOI] [PubMed] [Google Scholar]
- 39.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brisbare-Roch C, et al. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- 41.Szklo-Coxe M, Young T, Finn L, Mignot E. Depression: Relationships to sleep paralysis and other sleep disturbances in a community sample. J Sleep Res. 2007;16:297–312. doi: 10.1111/j.1365-2869.2007.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lutter M, et al. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008;28:3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson's disease. Brain. 2007;130:1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Comella CL. Sleep disorders in Parkinson's disease: An overview. Movement Disorders. 2007;22:S367–S373. doi: 10.1002/mds.21682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.