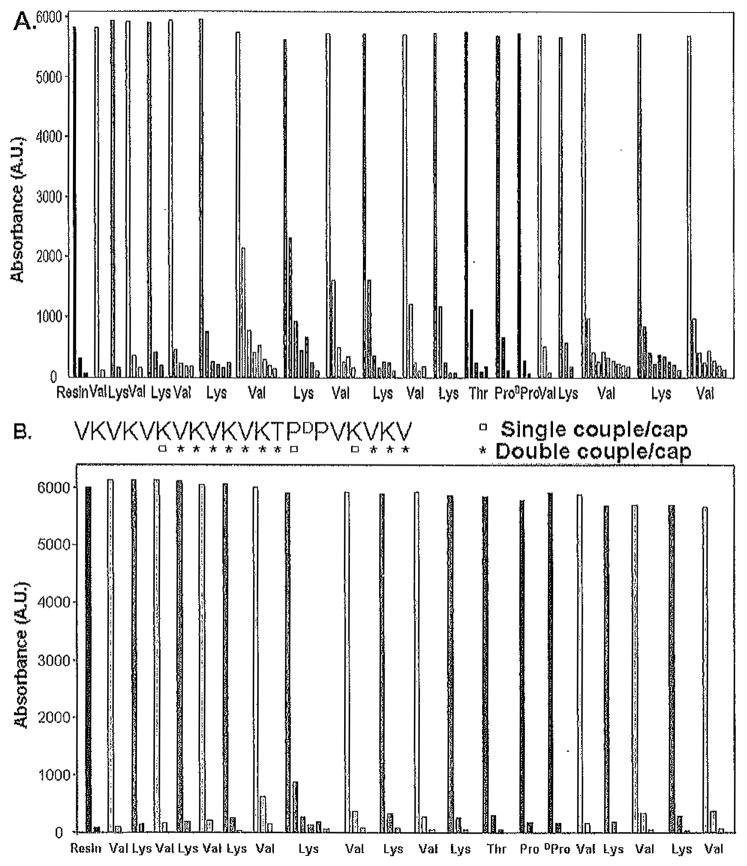
Fig. 1.
Profiles monitoring UV absorbance of the dibenzofulvene-piperidine adduct at 301 nm for each residue in the synthesis of the peptide VKVKVDPPTKVKVKVKVKVKV-NH2. (A) Nonoptimized synthesis in which each residue is single coupled, and 20% piperidine in N-methylpyrrolidone (NMP) is used for deprotection. (B) Optimized synthesis in which amino acid double coupling and N-terminal capping with acetic anhydride are employed at the positions indicated in the sequence (written from C- to N-terminus as synthesized). In addition, 1% 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) and 19 % piperidine in NMP is employed for 9-fluorenylmethoxycarbonyl (Fmoc) deprotection in panel B.