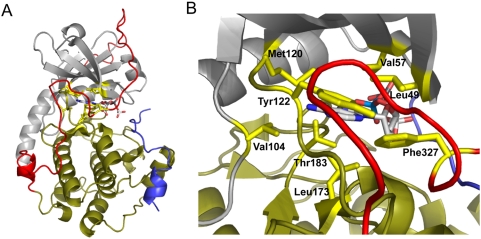
Figure 1. Residues highlighted to interact with the nucleotide base in PKA.
When PKA is in an ATP-bound state, the small lobe (gray) and glycine-rich loop move toward the large lobe (sand) to enclose the nucleotide-binding pocket around ATP. A, A global view of PKA in a nucleotide-bound state. Eight residues have been identified to interact with the nucleotide base: Leu 49, Val 57, Val 104, Met 120, Tyr 122, Leu 173, Thr 183, and Phe 327 (highlighted yellow). The bound nucleotide is represented as a ball-and-stick figure. The C-terminal tail is colored red, and PKI is colored magenta. B, Zoomed view into the nucleotide binding pocket. The nucleotide binding pocket is largely preformed with the exception of Val 57, Met 120, Leu 173, and Phe 327 which undergo structural changes upon nucleotide binding. Structure adapted from PDB accession number 1ATP and modeled using Pymol.