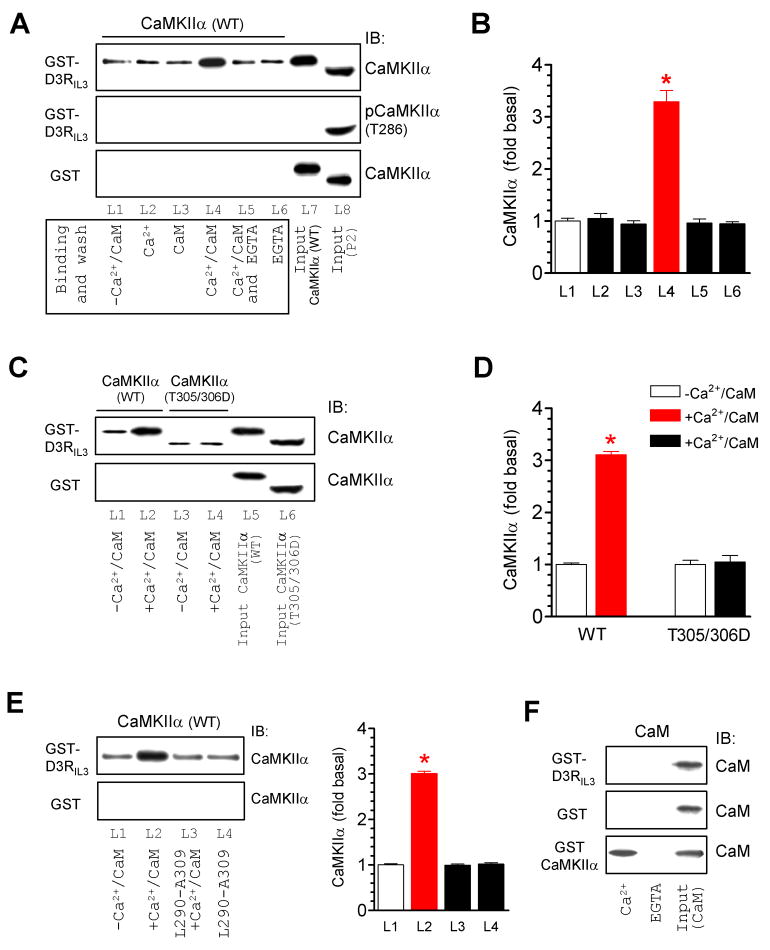
Figure 2. Ca2+/CaM-regulated CaMKIIα binding to D3RIL3.
(A and B) Addition of Ca2+/CaM increased the CaMKIIα binding to GST-D3RIL3. Note that, in the middle panel of Fig. 2A, CaMKIIα was not autophosphorylated at T286 in binding assays lacking ATP (L1-L6) as opposed to T286-autophosphorylated CaMKIIα detected in the synaptosomal fraction (P2) from the rat NAc (L8) with a phospho-specific antibody. (C and D) Addition of Ca2+/CaM did not alter the binding of CaMKIIα T305/306D mutant to GST-D3RIL3. (E) Co-addition of an inhibitory peptide (L290-A309) prevented an increase in the CaMKIIα-D3RIL3 binding induced by Ca2+/CaM. (F) CaM did not bind to GST-D3RIL3 or GST whereas it bound to GST-CaMKIIα in a Ca2+-dependent manner. Binding assays were performed between His-tagged WT CaMKIIα (~57 kDa), T305/306D mutant (~50 kDa), or CaM proteins and immobilized GST, GST-D3RIL3, or GST-CaMKIIα in the presence or absence of CaCl2 (0.5 mM), CaM (1 μM), EGTA (1 mM), or L290-A309 (5 μM). Data are presented as means ± SEM for 3–6 experiments per group. *p < 0.05 versus L1.