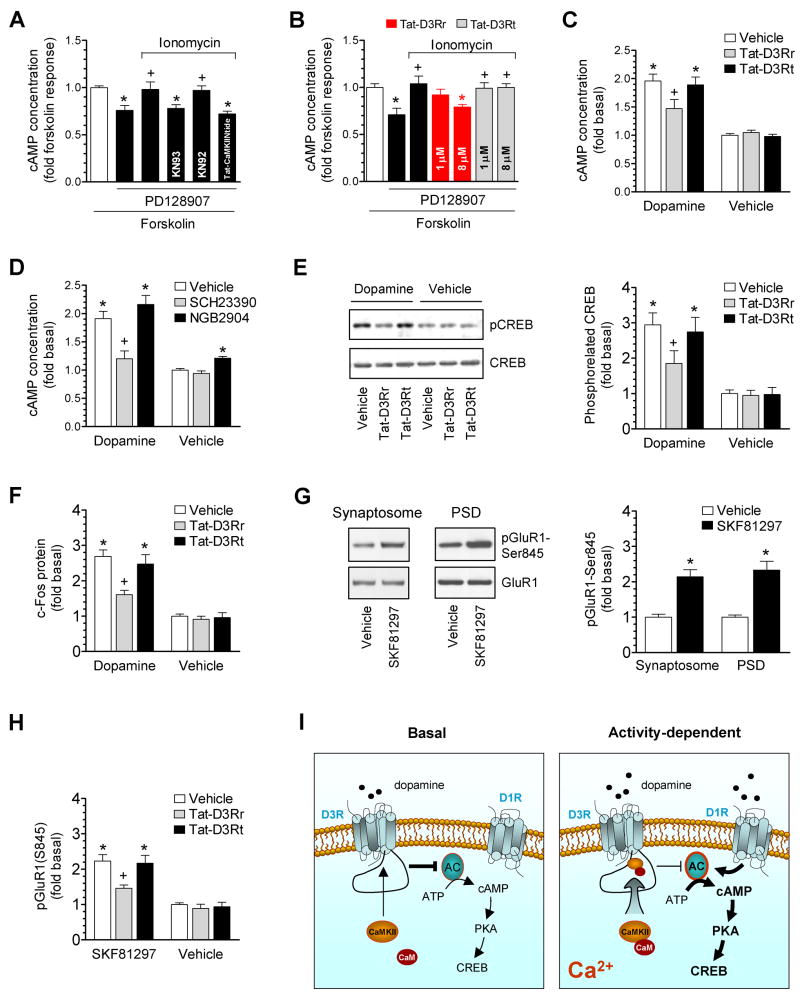
Figure 7. Inhibition of D3R function by CaMKII in rat accumbal slices.
(A) Ionomycin antagonized the D3R-mediated inhibition of forskolin-stimulated cAMP accumulation. (B) Tat-D3Rr reversed the effect of ionomycin. (C) Effects of Tat-fusion peptides on dopamine-stimulated cAMP accumulation. (D) Effects of the D1R or D3R antagonist on dopamine-stimulated cAMP accumulation. (E) Effects of Tat-fusion peptides on dopamine-stimulated CREB phosphorylation. (F) Effects of Tat-fusion peptides on dopamine-stimulated c-Fos expression. (G) SKF81297 increased pGluR1-S845 levels. (H) Effects of Tat-fusion peptides on SKF81297-stimulated GluR1 phosphorylation. (I) A model of the Ca2+-dependent regulation of D3Rs by CaMKII. AC, adenylyl cyclase. PD128907 (1–3 nM), ionomycin (10 μM with 1 mM CaCl2), KN93 (20 μM), KN92 (20 μM), SCH23390 (1 μM), and/or NGB2904 (50 nM) were co-treated with forskolin (1 μM, 20 min) or dopamine (5 μM, 20 min) in A–D. In the experiments in which the Tat-fusion peptide (Tat-CaMKIINtide, Tat-D3Rr, or Tat-D3Rt) was employed, the peptide at 5 μM (A), 1 and 8 μM (B), or 8 μM (C, E, F, and H) was applied 1 h prior to forskolin (A and B), dopamine (C, E, and F), or SKF81297 (H). SKF81297 was incubated at 10 μM for 5–10 min (G and H). Data are presented as means ± SEM for 5–7 experiments per group. *p < 0.05 versus forskolin alone (A and B), vehicle (G), or vehicle + vehicle (C–F and H). +p < 0.05 versus PD128907 + forskolin (A and B), vehicle + dopamine (C–F), or vehicle + SKF81297 (H).