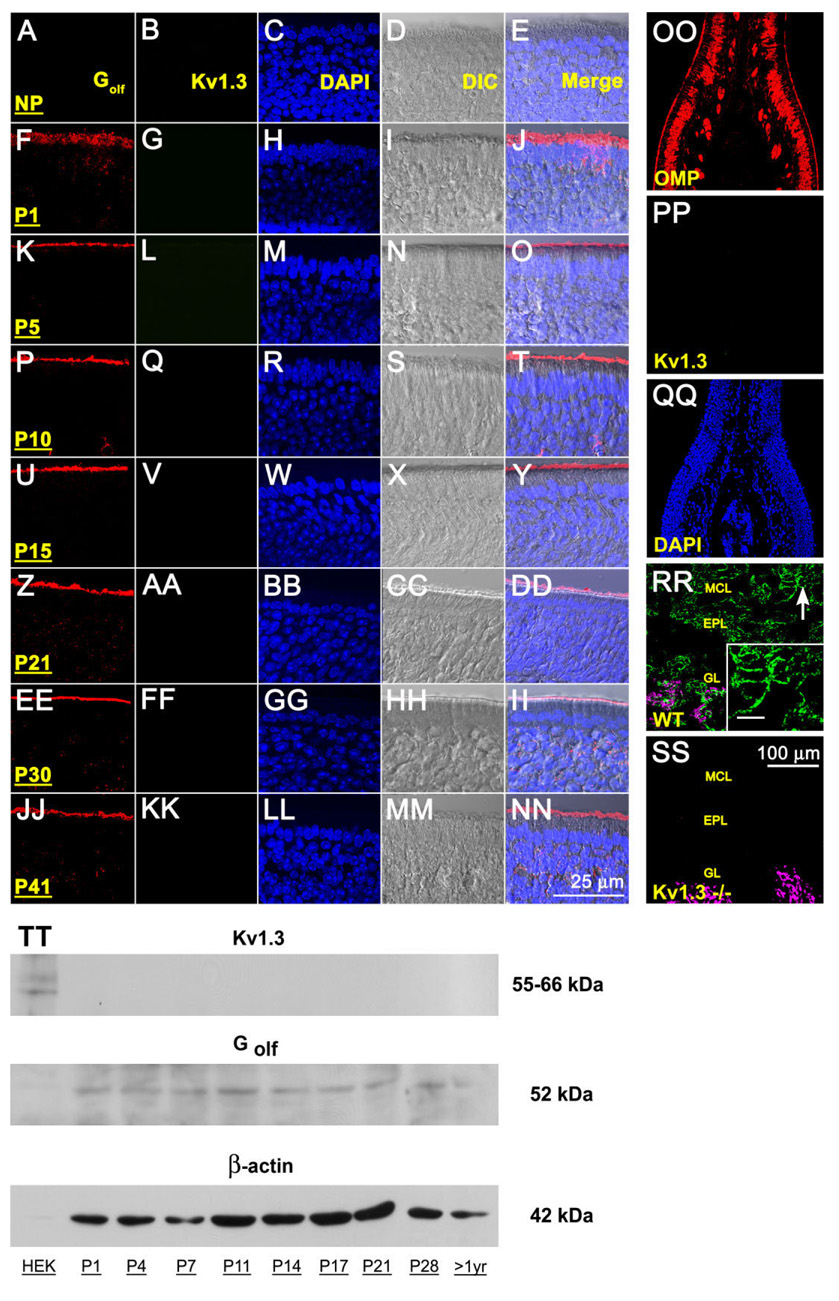
Fig. 1.
Lack of Kv1.3 ion channel protein expression in the main olfactory epithelium (MOE) by two different immunodetection approaches. A-NN: Representative photomicrographs of 16-µm coronal cryosections of the sensory epithelium processed for αKv1.3 (1:500) and α-Golf (1:500) immunoreactivity over a series of postnatal stages at high magnification. P1–P41, postnatal (P) days; NP, no primary control. Representative for P41. Column 1 (Golf), red channel; column 2 (Kv1.3), green channel; column 3 (DAPI, nuclear stain), blue channel; column 4 (DIC [differential interference contrast]), brightfield image; column 5 (merge), overlay of A–D, respectively. Wild-type B6C57 mice (OR untagged mice). OO–SS: Representative photomicrographs as above but processed for αKv1.3 (1:500) and/or αOMP (1:1,000) immunoreactivity at lower magnification to be inclusive of other cell types in the epithelium (OO–QQ) or the olfactory bulb (RR–SS). OO–QQ: Note absence of detection of Kv1.3 protein (green channel) but positive immunolabeling for another marker known to detect mature olfactory neurons (OMP; red channel) and axonal processes in the MOE. RR–SS: αOMP immunoreactivity (red channel; converted magenta) is intertwined but non-overlapping with the αKv1.3 signal in the glomerular layer (GL). Arrow, mitral cell body and processes labeled with αKv1.3; Inset: Higher magnification of mitral cell layer (MCL) and associated processes. Note positive immunolabeling for Kv1.3 protein in the olfactory bulb of wild-type B6C57 mice (WT) but absence of detection in mice with Kv1.3-gene-targeted deletion (Kv1.3−/−). EPL, external plexiform layer. Postnatal day 41. TT: Representative Western blot in which 25 µg of purified epithelial membrane proteins were separated by SDS-PAGE and electro-transferred to nitrocellulose for wild-type B6C57 mice. Proteins were harvested at various postnatal stages as noted. Nitrocellulose was blotted with a polyclonal antibody against Kv1.3 (αKv1.3; 1:1,000) with Mr = 55–66 kDa (depending on phosphorylation state) and then stripped and reprobed with anti-β-actin (α-β-actin; 1:1,000) to confirm equal loading of protein. Cloned Kv1.3 channel protein, heterologously expressed in HEK293 cells (HEK) and prepared as a cell lysate, was used as a positive migration standard. Labeling with an antibody to another membrane-bound protein (α-Golf; 1:1,000) known to be enriched in MOE membranes was used as an additional positive control. Scale bar = 25 µm in NN (applies to A–NN) 100 µm in SS (applies to OO–SS); 10 µm in inset to RR.