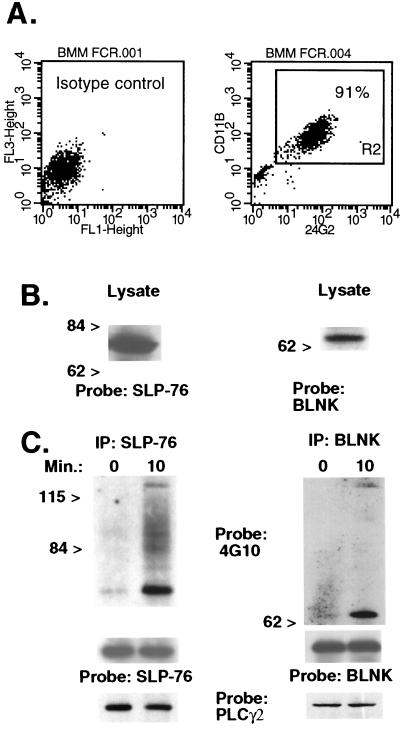
Figure 1.
SLP-76 and BLNK are expressed in BMM and tyrosine-phosphorylated after FcγR crosslinking. (A) BMM were incubated with labeled isotype control antibodies (Left) or FITC-conjugated 2.4G2 (anti-FcγRII/III) and biotinylated rat anti-mouse CD11b followed by streptavidin-cytochrome c (Right). The percentage of double-positive cells ranged from 90% to 95% in three separate experiments using different mice. (B) A Western blot of BMM lysates was probed with purified goat anti-SLP-76 or rabbit anti-BLNK antiserum. (C) BMM were exposed to 2.4G2 followed by goat anti-rat IgG for 0 or 10 min. Lysates were immunoprecipitated (IP) by using the anti-SLP-76 H3 murine mAb or rabbit anti-BLNK antiserum. A Western blot was probed initially with antiphosphotyrosine (4G10). The membrane was stripped and reprobed with goat anti-SLP76 or rabbit anti-BLNK to verify equal protein loading. In separate experiments, Western blots of SLP-76 and BLNK immunoprecipitates were hybridized with rabbit anti-human PLC-γ2. Results were identical in three separate experiments using different mice.