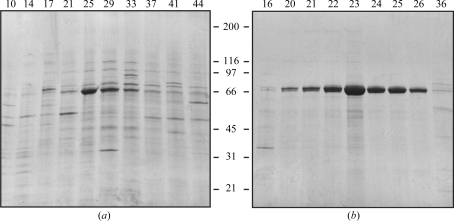
Figure 1.
Purification of rhTGFBIp using heparin-affinity chromatography. (a) Reducing SDS–PAGE of selected fractions separated by heparin-affinity chromatography. TGFBIp migrates as a clear band at approximately 65 kDa. Fractions 23–27 were pooled for further purification. (b) Reducing SDS–PAGE of selected fractions from anion-exchange chromatography. TGFBIp eluted around fraction No. 23 with a purity of approximately 95% (by visual estimation). Fractions 22–27 were pooled and used for crystallization. Fraction numbers are indicated at the top of the gels and molecular weights (in kDa) are indicated at the side.