Abstract
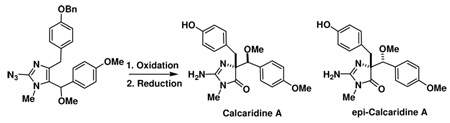
The first total synthesis of the Leucetta alkaloid calcaridine A is described based on a biosynthetic postulate. Application of an oxidative rearrangement of a 4,5-disubstituted imidazole leads to the formation of both calcaridine A and epi-calcaridine A. An X-ray crystal structure determination on the latter has allowed the assignment of the relative configuration of the epimeric natural product and calcaridine A by extrapolation.
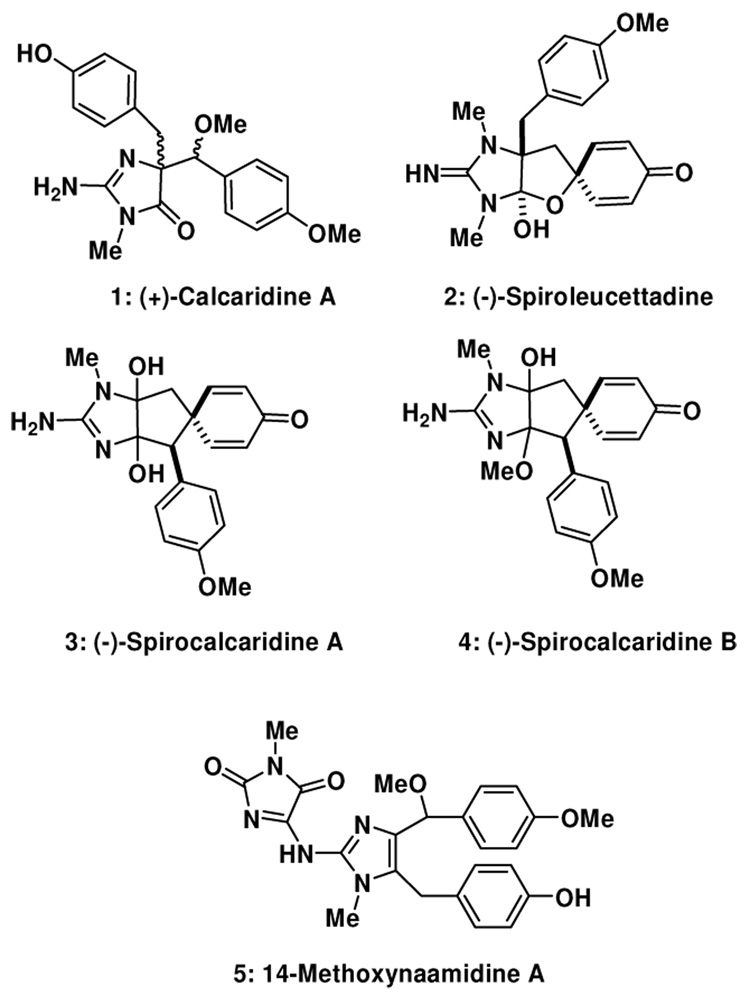
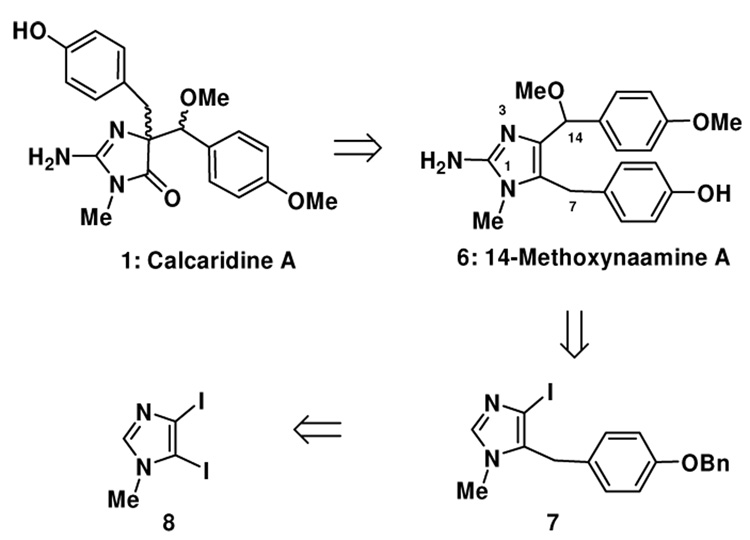
2-Aminoimidazole-containing alkaloids isolated from marine sponges have recently captured the attention of a number of synthetic groups, in particular several members of the oroidin family.1–6 Other families of imidazole-containing alkaloids, including examples isolated from Leucetta sponges, have similarly attracted the attention of several groups.7–9 Recently Crews and coworkers reported a series of structurally novel natural products 1–4 isolated from a Fijian marine sponge, Leucetta sp., some of which have modest anti-bacterial activity.10, 11 Some concerns have been raised based on synthetic studies directed towards spiroleucettadine (2) with respect to the accuracy of the assigned structure.12–14 This structural ambiguity notwithstanding, within each of these compounds a 2-aminoimidazole moiety and two benzyl moieties can be identified, although there are differences in the position of oxidation. There are obvious, if experimentally undefined, biosynthetic relationships between these molecules, and thus an approach to one may provide intermediates that can be used en route to other family members. In both the Crews reports,11 and in a subsequent report by Watson and co-workers of an approach to the assigned structure of spiroleucettadine (2),12 it is suggested that calcaridine A (1) and the other congeners 2–4 are derived from rearrangement and/or oxidation chemistry of naamine A15–17 (14-desmethoxy 6) or a closely related derivative.10–12 This particular postulate led us to speculate that calcaridine A, at least, is formed by an oxidative rearrangement of an intermediate that we term 14-methoxynaamine A (6). Although 6 appears to be unknown in the literature at the present time, several years ago the N-substituted derivative, 14-methoxynaamidine A (5) was isolated from a Leucetta sp. sponge, and therefore it is tempting to speculate that 6 is a biosynthetic precursor to both (1) and (5).18 In this communication, we describe the successful execution of a “biomimetically-inspired” approach to (±)-calcaridine A (1) which employs our recently discovered oxidative rearrangement of 4,5-disubstituted imidazoles to the corresponding 4,4-disubstituted-5-imidazolone.19–21 Although calcaridine A may not be considered a complex and challenging natural product per se, it contains two vicinal stereocenters for which neither the absolute nor the relative stereochemistry were reported (our synthetic studies have led to the assignment of the relative stereochemistry). In addition, in the isolation reports no description of the biological activity of this natural product was provided and these endeavors should provide sufficient material to facilitate such an investigation.
We have for some time had an interest in the development of new methods and strategies for the construction of complex imidazole-containing natural products from simple imidazole derivatives, rather than de novo synthesis of the imidazole ring.3 Towards this end, we have found that 4,5-disubstituted imidazoles on reaction with dimethyldioxirane or N-sulfonyloxaziridines undergo a rapid rearrangement to 5-imidazolones, or in some cases oxidative additions.19, 21, 22 On the basis of these discoveries, we believed that these reactions might prove useful en route to the Leucetta alkaloids 1–4 depicted in Figure 1.10, 11 However, prior to the work described herein, all of the reported examples are based on oxidation of tetrahydrobenzimidazole derivatives, therefore if the rearrangement reaction were to be employed in the present setting, this would suggest 6 as a precursor, constituting a new class of substrates for this chemistry (Figure 2). This rearrangement substrate would then be accessible from the corresponding 4,5- diiodoimidazole via a sequence of position selective metallation reactions, which based on our own work22–24 and others25–29 it is known that this can be executed in the order 5-, 4- and then 2-positions.
Figure 1.
Selected Leucetta alkaloids
Figure 2.
Retrosynthetic analysis of Calcaridine A
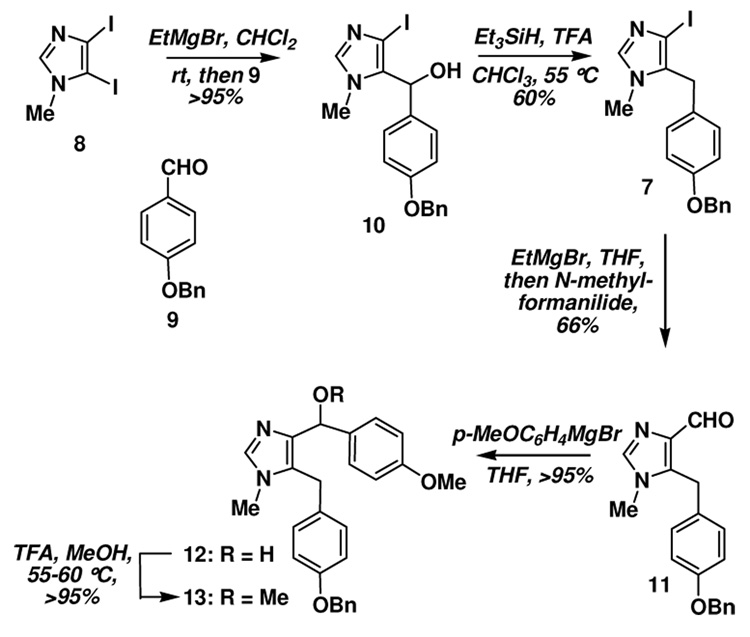
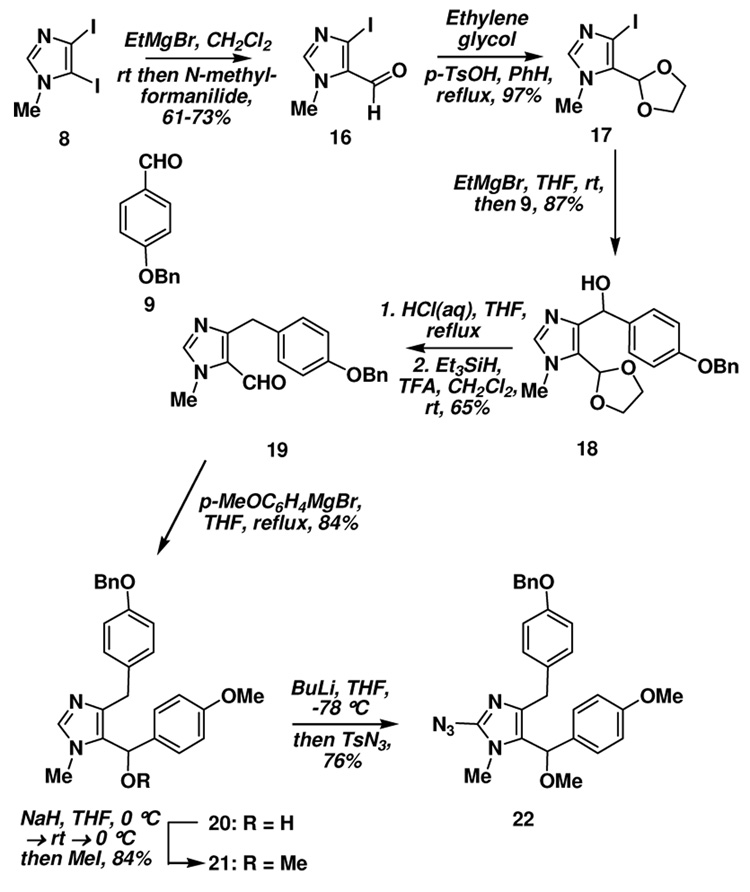
Our initial studies centered on the alkylation of diiodoimidazole 8 at the 5-position; this was attempted first by metallation (I → MgBr), transmetallation (MgBr → CuX) and then treatment with the appropriate benzyl bromide derivative.26 Unfortunately, the desired product was not obtained in this case, but rather the imidazolium salt was isolated. It was subsequently determined that the required adduct could be prepared by reaction of the in situ generated 5-imidazolyl Grignard with Bn-protected benzaldehyde derivative 9 and ionic reduction of the hydroxyl moiety in 10 which provided 7 (Scheme 1).8 Initial attempts to install the second benzylic substituent via similar metallation chemistry and reaction with anisaldehyde were unsuccessful. However metallation and reaction with N-methylformanilide gave the corresponding aldehyde 11 in good yield, which on reaction the Grignard reagent derived from bromoanisole provided the desired alcohol 12. Methylation of 12 was readily accomplished on exposure of 11 to methanol in the presence of trifluoroacetic acid.
Scheme 1.
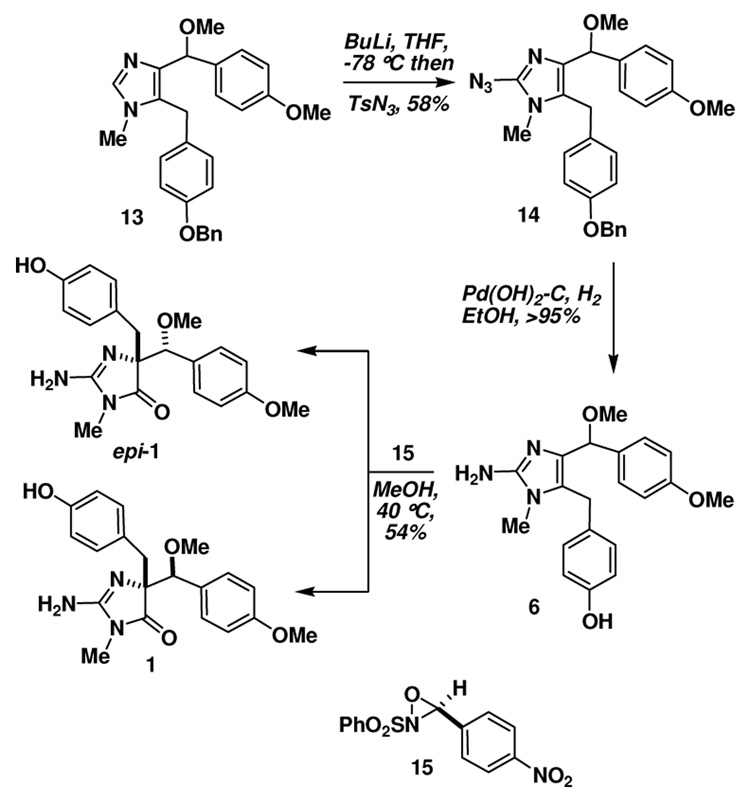
Introduction of the 2-amino group was carried out by C2-lithiation (n-BuLi) and reaction with TsN3 (Scheme 2).30 Simultaneous reduction of the azide and debenzylation was accomplished with Pd(OH)2-C/H2 providing the requisite rearrangement substrate 6 in good yield. We were delighted to discover that this substrate underwent oxidative rearrangement upon treatment with an N-sulfonyloxaziridine (15) in MeOH at 40 °C.19, 31 The reaction proceeded in a modest 54% yield (unoptimized), but unfortunately produced the rearrangement products 1 and epi-1 as 2:1 mixture of diastereomers based on 1H NMR spectroscopy, favoring the epimeric natural product. This problem was exacerbated by virtue of the difficulty encountered during attempted chromatographic separation of the two diastereomers.32
Scheme 2.
As a result of the poor diastereoselectivity (and the subsequent purification issues), we decided to modify our approach by interchanging the locus of the two benzylic fragments with the idea that the presence of the chiral center closer to the 5-position, where the oxygen transfer occurs, should lead to improved diastereoselectivity. The construction of the requisite substrate again began from 8 by metallation and reaction with N-methylformanilide (Scheme 3), providing the aldehyde 16 in good yield. The aldehyde was converted to the ethylene ketal 17 and then metallation and treatment with the benzyl-protected benzaldehyde derivative 9 provided 18. Alcohol 18 was deprotected with aqueous HCl and subjected to an ionic reduction of the doubly benzylic alcohol with Et3SiH/TFA providing aldehyde 19. A subsequent Grignard reaction with p-anisylmagnesium bromide gave alcohol 20, which was then converted to the methyl ether 21 (NaH, MeI). Introduction of the azide via metallation at C2 and trapping with TsN3 afforded 22 (Scheme 3).
Scheme 3.
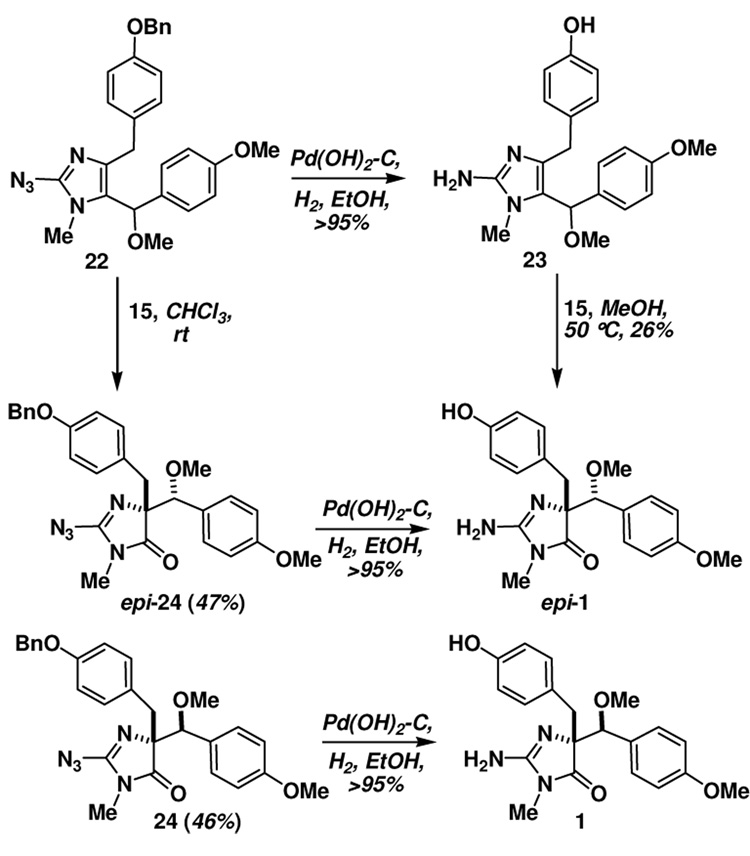
Exposure of 22 to Pd(OH)2-C/H2 led to reduction of both the azide and the hydrogenolysis of the O-benzyl moiety, providing the key rearrangement substrate 23 (Scheme 4). Treatment of 23 with the N-sulfonyloxaziridine 15 also led to an oxidative rearrangement, providing a 1:1 mixture of calcaridine A (1) and epi-calcaridine A epi-(1). Although there was a small change in the diastereoselectivity in favor of the natural product, it was certainly not to the extent that we had hoped and expected. At this point, we decided to investigate whether rearrangement of a precursor, in particular 22, might led to improved selectivities and facilitate separation of the epimeric imidazolones. We were delighted to discover that azide 21 underwent rearrangement on treatment with 15 to provide a 1:1 mixture of chromatographically separable imidazolones 24 and epi-24. At this point we did not know for certain which of the two azides possessed the appropriate relative stereochemistry for conversion to calcaridine A, although one had a strikingly similar NMR spectrum to 1. Therefore, each of these rearrangement products was taken on individually through the next step which involved catalytic hydrogenation, one of which led cleanly to calcaridine A (1), and the other to epi-calcaridine A epi-(1). Synthetic and natural calcaridine A exhibited identical NMR data, however our synthetic material was isolated as a solid, whereas the natural material was isolated as an oil, presumably this reflects differences of scale, purity or perhaps the fact that the synthetic material is racemic.
Scheme 4.
During the characterization process it was noted that epi-calcaridine A epi-(1) formed nice crystals from MeOH-D4, one of which was subjected to X-ray crystallography, which indicated a syn arrangement of the imidazolone nitrogen and the methoxy moiety.33 The structure clearly confirms that the rearranged product has the correct connectivity expected from this sequence, and provides the relative configuration of the two chiral centers. This in turn allowed the assignment of the relative configuration of calcaridine A (1) as indicated in Scheme 4.
The present report constitutes the first description of a total synthesis of the Leucetta alkaloid calcaridine A which employs an oxidative rearrangement of a polysubstituted imidazole. These are the first examples of rearrangements involving substrates other than a tetrahydrobenzimidazole-type derivative. While these experiments do not prove that calcaridine A is formed biosynthetically via an oxidative rearrangement of 14-methoxynaamine A, they do demonstrate that it is a feasible pathway. Although chemically the rearrangement exhibits poor diastereoselectivity, an enzyme-mediated process might be expected to proceed with substantially higher levels of selectivity. In addition to the total synthesis, we have determined the relative stereochemistry of the natural product through X-ray crystallography of the epimeric congener. Present efforts are directed toward improving the diastereoselectivity, developing an asymmetric synthesis and investigating the biological activity of this natural product and its epimer.
Supplementary Material
Supporting Information Available Detailed experimental procedures and copies of 1H and 13C NMR spectra for all new compounds. The X-ray structure of epi-(1) and associated CIF.
Acknowledgements
This work was supported by the Robert A. Welch Foundation (Y-1362) and the NIH (GM065503). The NSF (CHE-9601771 and CHE-0234811) is thanked for partial funding of the purchase of the NMR spectrometers used in this study.
References
- 1.Arndt H-D, Riedrich M. Angew. Chem., Int. Ed. 2008;47:4785. doi: 10.1002/anie.200801793. [DOI] [PubMed] [Google Scholar]
- 2.Weinreb SM. Nat. Prod. Rep. 2007;24:931. doi: 10.1039/b700206h. [DOI] [PubMed] [Google Scholar]
- 3.He Y, Du H, Sivappa R, Lovely CJ. Synlett. 2006:965. [Google Scholar]
- 4.Jacquot DEN, Lindel T. Curr. Org. Chem. 2005;9:1551. [Google Scholar]
- 5.Blunt JW, Copp BR, Hu W-P, Munro MHG, Northcote PT, Prinsep MR. Nat. Prod. Rep. 2008;25:35. doi: 10.1039/b701534h. [DOI] [PubMed] [Google Scholar]
- 6.Morris JC, Phillips AJ. Nat. Prod. Rep. 2008;25:95. doi: 10.1039/b701533j. [DOI] [PubMed] [Google Scholar]
- 7.Zhong J. Nat. Prod. Rep. 2005;22:196. doi: 10.1039/b316104h. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura S, Kawasaki I, Kunimura M, Matsui M, Noma Y, Yamashita M, Ohta S. J. Chem. Soc., Perkin Trans. 1. 2002:1061. [Google Scholar]
- 9.Kawasaki I, H N, Yanagitani S, Kakuno A, Yamashita M, Ohta S. J. Chem. Soc., Perkin Trans. 1. 2001:3095. [Google Scholar]
- 10.Edrada RA, Stessman CC, Crews P. J. Nat. Prod. 2003;66:939. doi: 10.1021/np020503d. [DOI] [PubMed] [Google Scholar]
- 11.Ralifo P, Crews P. J. Org. Chem. 2004;69:9025. doi: 10.1021/jo048789+. [DOI] [PubMed] [Google Scholar]
- 12.Aberle N, Ovenden SPB, Lessene G, Watson KG, Smith BJ. Tetrahedron Lett. 2007;48:2199. [Google Scholar]
- 13.Chang JJ, Chan B, Ciufolini MA. Tetrahedron Lett. 2006;47:3599. [Google Scholar]
- 14.Li C, Danishefsky SJ. Tetrahedron Lett. 2006;47:385. [Google Scholar]
- 15.Carmerly S, Kashman Y. Tetrahedron Lett. 1987;28:3003. doi: 10.1016/S0040-4039(00)96754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmely S, Ilan M, Kashman Y. Tetrahedron. 1989;45:2193. [Google Scholar]
- 17.Ohta S, Tsuno N, Nakamura S, Taguchi N, Yamashita M, Kawasaki I, Fujieda M. Heterocycles. 2000;53:1939. [Google Scholar]
- 18.Mancini I, Guella G, Debitus C, Pietra F. Helv. Chim. Acta. 1995;78:1178. [Google Scholar]
- 19.Sivappa R, Koswatta P, Lovely CJ. Tetrahedron Lett. 2007;48:5771. doi: 10.1016/j.tetlet.2007.06.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sivappa R, Hernandez NM, He Y, Lovely CJ. Org. Lett. 2007;9:3861. doi: 10.1021/ol0711568. [DOI] [PubMed] [Google Scholar]
- 21.Lovely CJ, Du H, He Y, Dias HVR. Org. Lett. 2004;6:735. doi: 10.1021/ol036403w. [DOI] [PubMed] [Google Scholar]
- 22.Lovely CJ, Du H, Sivappa R, Bhandari MK, He Y, Dias HVR. J. Org. Chem. 2007;72:3741. doi: 10.1021/jo0626008. [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Dias HVR, Lovely CJ. Tetrahedron Lett. 2003;44:1379. [Google Scholar]
- 24.Chen Y, Ekanayake V, Lovely CJ. Heterocycles. 2007;74:873. [Google Scholar]
- 25.Carver DS, Lindell SD, Saville-Stones EA. Tetrahedron. 1997;53:14481. [Google Scholar]
- 26.Yang X, Knochel P. Chem. Commun. 2006:2170. doi: 10.1039/b603419e. [DOI] [PubMed] [Google Scholar]
- 27.Abarbri M, Thibonnet J, Bérillon L, Dehmel F, Rottländer M, Knochel P. J. Org. Chem. 2000;65:4618. doi: 10.1021/jo000235t. [DOI] [PubMed] [Google Scholar]
- 28.Dehmel F, Abarbri M, Knochel P. Synlett. 2000:345. doi: 10.1021/jo000235t. [DOI] [PubMed] [Google Scholar]
- 29.Knochel P, Dohle W, Gommermann N, Kneisel F, Kopp F, Korn T, Sapountzis I, Vu V. Angew. Chem. Int. Ed. 2003;42:4302. doi: 10.1002/anie.200300579. [DOI] [PubMed] [Google Scholar]
- 30.Kawasaki I, Taguchi Y, Yamashita M, Ohta S. Heterocycles. 1996;43:1375. [Google Scholar]
- 31.Attempts to conduct the rearrangement with DMDO were unsuccessful leading to the formation of complex reaction mixtures
- 32.It was subsequently determined that the diastereomers could be separated by fractional recrystallization by seeding the mixture with the non-naturally occurring diastereomer
- 33.For details of this determination, see Supplementary Information
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Available Detailed experimental procedures and copies of 1H and 13C NMR spectra for all new compounds. The X-ray structure of epi-(1) and associated CIF.