Abstract
Gentamicin, an aminoglycoside with broad antimicrobial activity, is commonly used in both obstetrics and gynecology. Traditional dosing regimens for gentamicin have called for 3 times daily dosing, but recent insights into the pharmacodynamics of the drug have led to multiple studies of once-daily dosing regimens. Many studies have demonstrated efficacy, safety, and economy of the 24-hour dosing interval, resulting in recommendations that this become the standard for aminoglycoside administration. However, because of the unique considerations for drug administration in pregnant and postpartum women, the once-daily dosing regimens have not been widely adopted. Additional studies in pregnant and postpartum women have demonstrated therapeutic noninferiority, no increase in adverse events, and significant cost savings with once-daily dosing versus 3 times daily dosing of gentamicin. We review the literature and present rationale based on multiple-controlled studies supporting single-daily dosing of gentamicin, 5λmg/kg/d actual body weight, for many common obstetrics-gynecology infections.
Keywords: dosing, gentamicin, infection
Pharmacology
Gentamicin is an aminoglycoside antibiotic with bactericidal activity against Gram-negative bacteria. Other members of this class include tobramycin, amikacin, netilmicin, kanamycin, streptomycin, and neomycin. All aminoglycosides act at the 30S bacterial ribosomal subunit inhibiting the synthesis of bacterial proteins. When gentamicin is combined with a cell wall-active agent such as penicillin or vancomycin, there is a potent synergistic effect on certain Gram-positive bacteria such as enterococci, streptococci, and staphylococci. Tobramycin, amikacin, and netilmicin can be used interchangeably with gentamicin for susceptible infections, and their pharmacokinetics are similar; however, gentamicin is often preferred secondary to its lower cost and broader provider experience with its use.1
Aminogylcosides are hydrophilic and highly lipophobic, causing them to be poorly absorbed from the gastrointestinal tract but rapidly distributed after administration intramuscularly (IM) or intravenously (IV). The peak concentration of a dose given IM is seen in 30 to 90 minutes; the peak is seen about 30 minutes after IV dosing. The volume of distribution is about 25% of lean body weight, which is roughly equivalent to the volume of extracellular fluid.1 Gentamicin is excreted almost exclusively by glomerular filtration, and very little is reabsorbed after filtration. Concentration of gentamicin in the renal cortex and the inner ear can be higher than that in plasma, resulting in selective toxicity in these organs. Toxicity of gentamicin correlates with the duration of exposure above a toxicity threshold. Thus, trough monitoring has traditionally been used to assess the toxicity risk, with lower trough levels indicating decreased chances of end organ damage. Nephrotoxicity and ototoxicity are the adverse effects most often associated with gentamicin and can occur when trough levels exceed 2λμg/mL. Because of the sensitive kinetics and renal excretion, dosing may need to be adjusted in renal impairment or in times of increased glomerular filtration rate, including pregnancy and the immediate postpartum period.1
Gentamicin exhibits concentration-dependent bacterial killing, meaning that a minimum peak level must be achieved to obtain bactericidal activity, and that killing efficiency is amplified by increases in peak concentration. The recommended starting total daily dosage of gentamicin is 5 to 7λmg/kg/d to reach a target peak level of 4 to 10λμg/mL with conventional 3 times daily dosing.1 For a 90% clinical response, the peak should be 8 to 10 times the mean inhibitory concentration of the target organism. Mean inhibitory concentration levels for commonly encountered pathogens range from 0.5λμg/mL for Escherichia coli to 32λμg/mL for Enterococcus faecalis.1 Thus, the higher the peak gentamicin level, the greater the bactericidal activity (Fig. 1). Aminoglycoside antibiotics exhibit a postantibiotic effect (PAE), which means that bacterial growth is suppressed for a period of time after administration of the antibiotic. Bacteria in the PAE are more susceptible to intracellular killing and phagocytosis by leukocytes, but they also show increased resistance to uptake of additional drug. Evaluation of these pharmacokinetic properties of aminoglycosides leads to trials of once-daily dosing. If the level of bactericidal activity is based on the peak but the toxicity correlates with high troughs, giving 1 large dose would increase the peak whereas decreasing the total time above the recommended trough level as compared with divided doses. Once-daily dosing also uses the entire duration of the PAE, leaving a long enough dosing interval to avoid bacterial resistance to drug uptake.3
FIGURE 1.
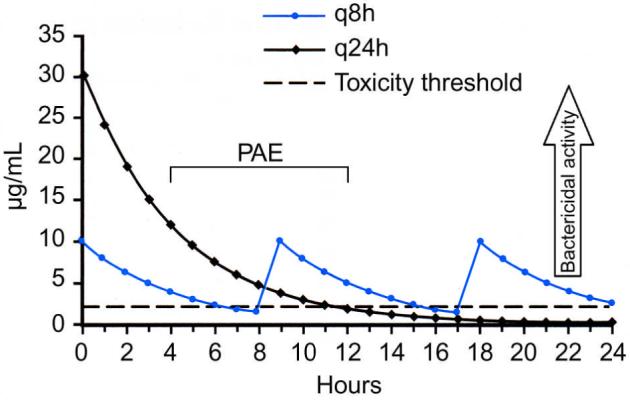
Plasma concentrations of 5λmg/kg of gentamicin as single-daily dosing or q8 hours. As peak levels increase, bactericidal activity increases. Notice the peak is nearly 3 times greater in once-daily dosing. The horizontal line represents 2λμg/mL, the trough level above which the risk of toxicity is increased. Note that with every 24-hour dosing, the time above the maximum trough is decreased. Also notice that the PAE (postantibiotic effect) overlaps the next dose of gentamicin in the every 8-hour regimen. (Adapted from Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 10th edn. NY: McGraw Hill; 2001:1219–1238).
Pregnancy and the Peripartum Period
PHYSIOLOGY
In pregnant and postpartum patients, glomerular filtration rate can be increased by 50% or more. The postpartum patient also has increased mobilization of fluid followed by diuresis as the body eliminates the increased fluid volume of pregnancy. Whereas the mean creatinine in the average nonobstetrical patient is 0.7λmg/dL, it is 0.5λmg/dL in the antepartum woman, and a value of 0.9λmg/dL or greater suggests renal disease in the pregnant patient.4 In a normal patient who is not pregnant or in the peripartum period, the half-life of gentamicin has been calculated to be 2.5 to 4 hours. In contrast, the half-life of gentamicin in peripartum patients can be as short as 1.4 to 1.8 hours. Studies measuring gentamicin clearance in postpartum patients after standard 3 times daily dosing have shown mean trough levels less than 2λμg/mL, and no patients had ototoxicity or nephrotoxicity. However, Zaske et al5 also found that 49% of patients studied required greater than the standard recommended dose to reach therapeutic peak levels. Briggs et al6 calculated a mean expected peak of 7.6λμg/mL; however, the observed mean in peripartum patients studied was 6.1λμg/mL with a subgroup of patients falling below the recommended peak of 6λμg/mL.
GENTAMICIN IN POSTPARTUM ENDOMETRITIS
Postpartum endometritis or simply metritis is infection of the uterus after delivery, usually by bacteria indigenous to the female genital tract. In studies that have cultured amniotic fluid at delivery, the most common organisms found were Peptostreptococcus, Peptococcus, Bacteroides, Clostridium, Enterococcus, group B Streptococcus, and E. coli. It is a clinical diagnosis characterized by fever, fundal tenderness, and foul-smelling lochia. Routine genital tract cultures are not recommended before treatment. Some risk factors include cesarean delivery, prolonged labor, prolonged rupture of membranes, multiple cervical examinations, and internal fetal monitoring. In patients undergoing cesarean section, antibacterial prophylaxis is the single most important intervention to prevent postpartum endometritis.4
Women with metritis after vaginal delivery will respond to ampicillin and gentamicin in 90% of the cases. Gentamicin is excreted in breast milk, but as it is poorly absorbed from the gastrointestinal tract, levels in exposed infants are not clinically significant, and no adverse effects have been found in breast-fed infants of mothers receiving gentamicin. In patients, status postcesarean section with metritis, the gold standard treatment is clindamycin 900λmg and gentamicin 1.5λmg/kg every 8λhours IV. Williams Obstetrics remarks that once-daily gentamicin dosing in this circumstance is an “acceptable” alternative. In suspected cases of sepsis or enterococcal infection, ampicillin should be added to the regimen.4
In a trial by Del Priore et al,7 patients with postpartum endometritis were randomized to gentamicin 5λmg/kg once-daily or 1.75λmg/kg 3 times daily, with both arms receiving clindamycin 900λmg IV every 8 hours. The authors found a significant decrease in the number of doses of both gentamicin and clindamycin, cost of administration, and nursing time in the experimental arm. There was no significant difference in length of stay or change in creatinine. Additionally, 24 out of 65 patients in the control arm needed dosage adjustments to reach therapeutic peak levels, compared to none of the experimental patients.7
Livingston et al,8 conducted a double-blinded, randomized, placebo-controlled trial comparing 2700λmg of clindamycin and 5λmg/kg of gentamicin once-daily to 900λmg of clindamycin and 1.5λmg/kg of gentamicin 3 times daily. They prospectively enrolled 110 patients with postpartum endometritis. The once-daily dosing arm showed significantly lower cost of administration. Although not statistically significant, a shorter course of antibiotics, shorter hospital stay, and fewer treatment failures were also seen in the experimental arm.8
Another study by Mitra et al9 examined patients treated for endometritis and patients with chorioamnionitis who were given postpartum antibiotics. Two hundred seventy-two patients were randomized to receive once-daily gentamicin and twice-daily clindamycin or traditional dosing. The authors found an equal cure rate for both regimens in patients who had chorioamnionitis. In patients with endometritis, the cure rate was 94.3% in the experimental group as compared with 87.6% in the control group; however, this difference was not significant when mode of delivery was considered. Again, a significant decrease in cost of administration was demonstrated with once-daily dosing.9 They concluded that once-daily gentamicin with twice-daily clindamycin was safe, as effective, and cost saving compared with the traditional regimen.
A smaller study evaluated pharmacokinetics in 10 patients receiving 4.5λmg/kg of gentamicin daily for postpartum endometritis. The authors measured trough levels 30λmin before the second and third dose and found all to be less than 0.3λμg/mL, which is well below the recommended &<2λμg/mL. The mean peak serum level on day 1 was 11.6λmg/L—well within the therapeutic range. The authors calculated a 44% decrease in cost of treatment compared with gentamicin administered 3 times daily. No toxicity was seen.10
GENTAMICIN IN CHORIOAMNIONITIS
Chorioamnionitis is an intrauterine infection during pregnancy. Like metritis, it is most often caused by bacteria indigenous to the genital tract. Risk factors include prolonged rupture of membranes, prolonged labor, multiple digital examinations, and internal fetal monitors. Standard treatment is with IV ampicillin and gentamicin.4 Gentamicin crosses the placenta, and umbilical cord sampling has found peak fetal levels to be 34% to 42% of maternal levels. As previously stated, there is increased clearance and a decreased half-life of gentamicin in pregnant women. However, clearance is decreased in preeclamptic patients and dosing must be adjusted according to peak and trough levels if three times daily dosing is used in those patients. No ototoxicity or nephrotoxicity has been documented in human fetuses exposed to gentamicin.
In a study by Locksmith et al,11 a single dose of 5.1λmg gentamicin/kg ideal body weight of was given to 18 laboring patients with a diagnosis of chorioamnionitis. The control arm of 20 laboring women with chorioamnionitis received a 120λmg load followed by 80λmg every 8 hours. The investigators found an inverse correlation between the fetal gentamicin level and the time of last maternal dosage and estimated the peak fetal level to be one-third the peak of maternal level. The median maternal peak level was 18.2λμg/mL in the once-daily group and 7.1λμg/mL in the conventional arm, and no patient in the experimental arm had a peak concentration less than 10λμg/mL. Extrapolated peak cord blood levels were 6.9λμg/mL when the mother received once-daily gentamicin and 2.9λμg/mL with conventional dosing. The time for gentamicin level of 2λμg/mL or less was 10 hours in the experimental group versus 5 hours in the conventional arm, giving total time below the toxicity threshold of 14 hours per day in the experimental group versus 9 hours in the control arm. This could correlate with reduced risk of nephrotoxicity and ototoxicity. The clearance of gentamicin by the fetus was similar to previous findings for neonates. There was no difference in maternal or fetal outcome between the 2 arms.11
GENTAMICIN FOR PYELONEPHRITIS IN PREGNANCY
Pyelonephritis can be a serious complication of pregnancy. The most common organisms isolated are E. coli, Klebsiella pneumonia, Enterobacter, and Proteus. Ampicillin and gentamicin is one of the recommended antibiotic regimens for pyelonephritis in pregnancy and is 95% effective in treating this infection.12 No studies have been conducted on once-daily dosing of gentamicin in pregnant women with pyelonephritis, but given the safety in pregnancy demonstrated by above studies of chorioamnionitis, combined with the efficacy shown in the other literature, we see no reason that once-daily gentamicin should not be used to treat pyelonephritis in pregnancy.
GYNECOLOGIC INFECTIONS
Very little research has been done on once-daily gentamicin dosing in the setting of gynecologic infections; however, there have been many trials in the internal medicine literature discussing the efficacy of once-daily aminoglycosides in various serious Gram-negative infections. Two meta-analyses, the first by Barza et al evaluated 21 randomized trials. The second by Hatala et al, compiling results of 13 randomized trials, compared single-daily dosing of aminoglycosides with conventional dosing in patients without renal impairment. In both studies, the patients were all immunocompetent adults with a variety of infectious processes requiring aminoglycoside antibiotics. Barza et al found a nonsignificant decrease in treatment failure, a significant decrease in nephrotoxicity, and no difference in ototoxicity in the single-daily dosing arm versus the conventional arm. Hatala et al found that both dosing regimens had equivalent cure rates. They also found decreased mortality and toxicity in the once-daily arm, although this did not reach statistical significance. In both meta-analyses, the authors noted decreased cost and decreased nursing time associated with the once-daily arm.13,14
PELVIC INFLAMMATORY DISEASE
Pelvic inflammatory disease (PID) is an inflammatory disease of the upper genital tract, which includes endometritis, salpingitis, tubo-ovarian abscess (TOA), and pelvic peritonitis. Over 99% of these infections are caused by organisms in the vagina and cervix ascending to the upper genital tract. Often sexually transmitted infections such as Chlamydia trachomatis and Neisseria gonorrhea are the insinuating organism, but PID is almost always polymicrobial. Cytomegalovirus, Mycoplasma hominis, Ureaplasma urealyticum, and normal vaginal flora have been cultured from infected sites.15
PID is usually diagnosed clinically but in some cases laparoscopy can be used to collect cultures and evaluate for salpingitis. The clinical criteria for diagnosis of PID as defined by Hager et alAQ1are abdominal tenderness with or without rebound tenderness, cervical and uterine motion tenderness, and adnexal tenderness with one of the following: Gram stain of the endocervix showing intracellular Gram-negative diplococci, fever, leukocytosis, purulent material from culdocentesis or laparoscopy, or abscess or inflammatory complex on examination or ultrasound. The most important goals of treatment of PID are resolution of symptoms and preservation of tubal function.4,15 The Centers for Disease Control (CDC) recommends treating any sexually active reproductive aged woman who has cervical motion tenderness, adnexal tenderness, or uterine tenderness.16
Mild-to-moderate PID can be treated on an outpatient basis with oral antibiotics. The following are indications for hospitalization, parenteral antibiotics, and possible surgical intervention: tuboovarian complex or abscess, pregnancy, adolescence, immunodeficiency, surgical emergency, nausea and vomiting, recent history of procedure to genital tract, inadequate response to outpatient therapy, peritonitis in upper abdominal quadrants, or presence of an intrauterine device.15 If parenteral antibiotics are deemed necessary, cefotetan or cefoxitin plus doxycycline (regimen A) or clindamycin and gentamicin (regimen B) can be used. The CDC recommends clindamycin 900λmg IV every 8 hours with gentamicin 2λmg/kg loading dose IV or IM with a maintenance dose of 1.5λmg/kg every 8 hours. The guidelines also state that although there are no trials of once-daily gentamicin dosing in PID, it is efficacious in similar situations and therefore single-daily dosing may be substituted.16 Regimen B is preferred for patients with abscess, infection with an intrauterine device, or infection after surgery or any procedure involving the upper genital tract, as it has better coverage for anaerobic infections and facultative Gram-negative rods.15
One study evaluated once-daily dosing of aminoglycosides compared with conventional schedules in patients with moderate-to-severe PID. This study was carried out in 2 parts, evaluating both amikacin and netilmicin. Patients were given tinidazole 0.8λg daily and ampicillin 2λg twice-daily along with the aminoglycoside. The authors found similar clinical efficacy in all study groups. No overt renal toxicity developed in any group, although both once daily arms had statistically significant decreases in phospholipiduria compared with the conventional dosing groups, suggesting a lower degree of subclinical nephrotoxicity with daily dosing. No ototoxicity developed in either amikacin group, but the once-daily arm of the netilmicin group had significantly less auditory derangement than did the 3 times daily group. Together, the study’s toxicity data suggest that single-daily dosing causes less accumulation of aminoglycosides in the kidney and inner ear, validating previous animal studies.17 Although this study did not evaluate gentamicin specifically, the rigorous incorporation of formal audiometric testing and measurement of phospholipiduria allowed for more sensitive assessment of toxicity differences between the conventional and daily dosing frequencies. Thus, in the setting of acute PID, once-daily aminoglycoside dosing seems to have lower toxicity and equal efficacy when compared with conventional schedules.
PELVIC ABSCESSES
No studies have evaluated once-daily gentamicin dosing in the setting of pelvic abscesses. TOAs often complicate PID, and abscesses of the vaginal cuff may occur after hysterectomy. The mainstay of treatment for both types includes broad-spectrum parenteral antibiotics and a common regimen consists of ampicillin, gentamicin, and clindamycin. However, larger abscesses (greater than 5λcm maximum diameter) often also require surgical intervention, as antibiotics alone have a response rate of only about 70%. Studies have suggested that response to antibiotics may be related to abscess size, with abscesses less that 5λcm requiring additional intervention less than 20% of the time. A retrospective study by Goharkhay et al18 found that patients with large TOAs who underwent computed tomography or ultrasound-guided drainage as primary intervention had a 100% response. All patients concomitantly received gentamicin and clindamycin. Of the patients who failed antibiotic therapy and underwent subsequent drainage, 90.5% of them responded to treatment. Another retrospective analysis of 27 abscesses (13 TOA, 14 postoperative) treated using transvaginal ultrasound-guided aspiration along with gentamicin and clindamycin showed resolution in 25 of 27 abscesses.19 Although no studies have specifically addressed the issue, based on strong safety and efficacy evidence in other patient populations, we recommend once-daily dosing of gentamicin, along with standard clindamycin administration for postoperative pelvic abscesses and TOA, In addition, drainage of abscesses greater than 5λcm diameter has been shown to improve response to antimicrobial therapy.
SUMMARY AND RECOMMENDATIONS
Once-daily dosing of gentamicin has been extensively scrutinized in the internal medicine literature, and has been found to be at least as efficacious as traditional dosing without increased risk of toxicity. Overwhelming evidence demonstrates that cost is significantly decreased with single-daily dosing of gentamicin. These findings have been validated in patients with postpartum endometritis and in pregnant women with choriamnionitis. The dosages studied in obstetrics and gynecologic patients were 4 to 5.1λmg/kg, with 5λmg/kg actual body weight being the most frequently used (Table 1). A single-daily dose of 5λmg/kg has been shown to be safe, clinically effective and cost saving in obstetric and gynecologic patients. We recommend its use in patients with chorioamnionitis, postpartum endometritis, pyelonephritis, PID, and postoperative infections when an aminoglycoside is part of the recommended regimen.
Table 1.
Comparison of Studies Evaluating Single-daily Dosing (SDD) of Gentamicin in Postpartum Endometritis.
| Study | Study Design | Population | Experimental arm | Control Arm | Clinical Response |
Mean Peak Level |
Toxicity | Cost anaylsis |
|---|---|---|---|---|---|---|---|---|
| Del Priore et al. (6) | Double-blinded randomized clinical trial |
Postpartum endometritis |
Gentamicin 5mg/kg actual body weight IV SDD + Clindamycin 900Mg IV q8hr |
Gentamicin 1.75 mg/kg IV q8hr + Clindamycin 900mg IV q8hr |
47/62 vs. 49/65 (P = NS) |
16.8 ± 4.6 μg/mL. vs. 5.2 ± 1.3 μg/mL (P= <0.001) |
none | Significant decrease in administration cost and nursing time |
| Livingston et al. (7) | Double-blinded randomized clinical trial |
Postpartum endometritis |
Gentamicin 5mg/kg IV SDD + Clindamycin 2700 mg IV SDD |
Gentamicin 140 mg load then 120 mg IV q8hr if <90kg, 160mg load then 140 mg IV q 8hr if> 90kg + Clindamycin 900mg IV q8hr |
45/55 vs. 38/55 (P=0.12) |
Not commented on |
none | Comment stating SDD is less expensive, no values listed |
| Mitra et al. (8) | Randomized clinical trial |
1) Postpartum endometritis 2) prophylaxis against endometritis in patients with chorioamnionitis |
Gentamicin 4mg/kg IV SDD + Clindamycin 1200 mg IV q12h |
Gentamicin 1.33 mg/kg IV q8hr + Clindamycin 800mg IV q8hr |
1) 66/70 vs 58/71 (P=0.02- NS if controlled for mode of delivery) 2) 61/65 vs. 62/66 (P = 1.00) |
12.8 mg/dL vs. 5.3 mg/dL (P= 0.0001) |
One patient in experimental group (1.1%) with transient rise in creatinine |
$250.79 per pt vs. $442.49 |
| Sunyecz et al. (9) | Clinical trial | Postpartum endometritis |
Gentamicin 4.5mg/kg actual body weight IV SDD + Clindamycin |
none | 9/10 with clinical cure, 1 required heparin |
11.6 ± 2.3 mg/L |
none | 44% decrease in cost vs. TID regimen for 72 hours |
Acknowledgments
Regan N. Theiler is supported by the National Institutes of Health Women’s Reproductive Health Research grant number NICHD5K12 HD001269-D9.
References
- [1].Chambers HF. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. [Accessed 10/4/07];Aminoglycosides. 2006 [Google Scholar]
- [2].Chambers HF. The aminoglycosides. In: Hardman JGL, Lee E, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 10th McGraw Hill; NY: 2001. pp. 1219–1238. [Google Scholar]
- [3].Craig W.Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men Clin Infect Dis 1998261–10.; quiz 11-12. [DOI] [PubMed] [Google Scholar]
- [4].Anonymous . Puerperal infection. In: Cunningham FL, Leveno KJ, Hauth JC, et al., editors. Williams Obstetrics. 22nd McGraw-Hill; NY: 2005. pp. 711–724. [Google Scholar]
- [5].Zaske D, Cipolle R, Strate R, et al. Rapid gentamicin elimination in obstetric patients. Obstet Gynecol. 1980;56:559–564. [PubMed] [Google Scholar]
- [6].Briggs G, Ambrose P, Nageotte M. Gentamicin dosing in postpartum women with endometritis. Am J Obstet Gynecol. 1989;160:309–313. doi: 10.1016/0002-9378(89)90431-6. [DOI] [PubMed] [Google Scholar]
- [7].Del Priore G, Jackson-Stone M, Shim E, et al. A comparison of once-daily and 8-hour gentamicin dosing in the treatment of postpartum endometritis. Obstet Gynecol. 1996;87:994–1000. doi: 10.1016/0029-7844(96)00054-3. [DOI] [PubMed] [Google Scholar]
- [8].Livingston J, Llata E, Rinehart E, et al. Gentamicin and clindamycin therapy in postpartum endometritis: the efficacy of daily dosing versus dosing every 8 hours. Am J Obstet Gynecol. 2003;188:149–152. doi: 10.1067/mob.2003.88. [DOI] [PubMed] [Google Scholar]
- [9].Mitra A, Whitten M, Laurent S, et al. A randomized, prospective study comparing once-daily gentamicin versus thrice-daily gentamicin in the treatment of puerperal infection. Am J Obstet Gynecol. 1997;177:786–792. doi: 10.1016/s0002-9378(97)70269-2. [DOI] [PubMed] [Google Scholar]
- [10].Sunyecz J, Wiesenfeld H, Heine R. The pharmacokinetics of once-daily dosing with gentamicin in women with postpartum endometritis. Infect Dis Obstet Gynecol. 1998;6:160–162. doi: 10.1002/(SICI)1098-0997(1998)6:4<160::AID-IDOG4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Locksmith G, Chin A, Vu T, et al. High compared with standard gentamicin dosing for chorioamnionitis: a comparison of maternal and fetal serum drug levels. Obstet Gynecol. 2005;105:473–479. doi: 10.1097/01.AOG.0000151106.87930.1a. [DOI] [PubMed] [Google Scholar]
- [12].Anonymous . Acute pyelonephritis. In: Cunningham FL, Leveno KJ, Hauth JC, et al., editors. Williams Obstetrics. Vol. 22. McGraw-Hill; NY: 2005. pp. 1096–1099AQ2. [Google Scholar]
- [13].Hatala R, Dinh T, Cook D. Once-daily aminoglycoside dosing in immunocompetent adults: a meta-analysis. Ann Intern Med. 1996;124:717–725. doi: 10.7326/0003-4819-124-8-199604150-00003. [DOI] [PubMed] [Google Scholar]
- [14].Barza M, Ioannidis J, Cappelleri J, et al. Single or multiple daily doses of aminoglycosides: a meta-analysis. BMJ. 1996;312:338–345. doi: 10.1136/bmj.312.7027.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Droegemueller W. Infections of the Upper Genital Tract. In: Stenchever MD, M W, Herbst AL, et al., editors. Comprehensive Gynecology. 4th Mosby; St Louis: 2001. pp. 707–740AQ3. [Google Scholar]
- [16].Goetz C.Treatment of STDs: updated guidelines from the CDC JAAPA 20072016, 18. [PubMed] [Google Scholar]
- [17].Ibrahim S, Derde M, Kaufman L, et al. Safety, pharmacokinetics and efficacy of once-a-day netilmicin and amikacin versus their conventional schedules in patients suffering from pelvic inflammatory disease. Ren Fail. 1990;12:199–203. doi: 10.3109/08860229009065564. [DOI] [PubMed] [Google Scholar]
- [18].Goharkhay N, Verma U, Maggiorotto F. Comparison of CT- or ultrasound-guided drainage with concomitant intravenous antibiotics vs. intravenous antibiotics alone in the management of tubo-ovarian abscesses. Ultrasound Obstet Gynecol. 2007;29:65–69. doi: 10.1002/uog.3890. [DOI] [PubMed] [Google Scholar]
- [19].Corsi PJ, Johnson SC, Gonik B, et al. Transvaginal ultrasound-guided aspiration of pelvic abscesses. Infect Dis Obstet Gynecol. 1999;7:216–221. doi: 10.1002/(SICI)1098-0997(1999)7:5<216::AID-IDOG2>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nahum GG, Uhl K, Kennedy DL. Antibiotic use in pregnancy and lactation: what is and is not known about teratogenic and toxic risks. Obstet Gynecol. 2006;107:1120–1138AQ4. doi: 10.1097/01.AOG.0000216197.26783.b5. [DOI] [PubMed] [Google Scholar]


