Abstract
Frailty is a state of health signified by an increased vulnerability to adverse health outcomes in the face of stressors (e.g. infection). There is emerging consensus that research on both the theory and measurement of frailty must focus on the dynamic interactions within and across systems underlying the frailty syndrome. In this paper, we propose a dynamical systems modeling approach, based on the stimulus-response experimental paradigm, to propel future advances in the study of frailty. Our proposal is novel in that it provides a quantitative framework to operationalize and test the core notion underlying frailty that it signifies a loss of resilience in homeostatic regulation. The proposed framework offers many important benefits, including (a) insights into whether and how homeostatic regulation differs between frail and non-frail older adults, (b) identification of critical regulatory systems, if they exist, that could function as sentinel systems for screening and early detection of frailty, (c) establishment of the value of provocative tests that can provide maximal information on the integrity of systems identified in (b), and (d) evaluation and unification of diverse empirical descriptions of frailty by providing a mathematical framework anchored in quantifying the loss of resilience, an essential property of frailty.
Keywords: frailty, dynamical systems, homeostasis, stimulus-response, provocative testing
A. Introduction
As our population ages, a central focus of geriatricians and public health practitioners is to understand, and then beneficially intervene on, the factors and processes that put elders in the community at elevated risk of catastrophic declines in health and function. The syndrome of frailty has been hypothesized to embody such risk, in particular the increased vulnerability to stressors (e.g. infection, injury, or even changes in medication) that characterizes many older adults [Fried et al, 2001]. We and others have theorized that this vulnerability results from dysregulation of interactions (e.g. impaired negative feedback) within and between multiple physiological regulatory functions in a complex adaptive system, leading to compromised ability to regulate homeostasis, or a loss of resilience in the face of stressors.[Lipsitz 2002; Fried et al, 2005].
Frailty research to date has focused on two areas: (1) the development and evaluation of empirical descriptions of geriatric frailty, based either on phenotypes [Fried et al, 2001] or on the manifested number of health-related deficits [Rockwood, Mitnitski, 2007]; and (2) the identification of biological markers associated with frailty (primarily from the phenotypical perspective). Phenotypical descriptions focus on functional manifestations of frailty involving dysregulated energetics, including muscle weakness, reduced exercise tolerance and/or energy, decreased walking speed, physical activity and weight loss [Fried et al, 2001], while deficit-based descriptions tabulate a broad range of “symptoms, signs, disabilities, diseases, and laboratory measurements” [Rockwood, Mitnitski, 2007]. These are important manifestations of frailty evidenced by findings that older individuals possessing one or more of them are at elevated risk for a range of adverse health outcomes including disability, admission into nursing homes, and mortality [Fried et al, 2001; Bandeen-Roche et al, 2006]. However, neither characterization has, yet, elucidated the core aspect of frailty, which is the mechanisms underlying vulnerability of the organism to stressors (e.g. [Lipsitz, 2002; Fried et al, 2005; Bergman et al, 2007]). It has been hypothesized that this vulnerability and its functional manifestations result from a critical accumulation of dysregulations in important signaling pathways involved in homeostatic regulation [Ferrucci et al, 2005; Fried et al 2005].
Studies have identified several biological markers (e.g. hormones, cytokines), measured under basal conditions, that are associated with the phenotypic manifestations of frailty (Walston et al.2002; Leng et al, 2004), and recent findings indicate that there is a non-linear relationship between numbers of biomarkers that are abnormal and likelihood of frailty (Fried et al., submitted). However, loss of resilience in homeostatic regulatory systems, which is theorized to underly the vulnerability to stressors of frailty, is fundamentally a dynamical construct. The theoretical literature on frailty has hypothesized that changes in the regulatory systems involved in the maintenance of homeostasis may well be subtle and undetectable in the absence of external stressors such as infection, injury, or organ-system based illness, and, rather, the frail and non-frail would differ more in terms of the dynamics of physiological systems in response to stimuli than they would in terms their baseline status [Buchner et al, 1992; Lipsitz, 2002]. Therefore “resilience” is a characteristic most observable in situations where an external stimulus induces measurable changes in the physiological system under study. Studying a biological system only under basal conditions by measuring static biomarkers cannot address the dynamic properties of that system, i.e. how the system would respond to a challenge. Nor does it acknowledge inter-person heterogeneity in basal levels independent of their functional status. Rather, stimulus-response studies of homeostatic regulatory systems have the potential to produce insights that will improve our understanding and treatment of the vulnerability associated with frailty. Accordingly, the recent AGS-NIA sponsored Research Agenda on Frailty [Fried et al, 2005] identified the development of dynamical systems approaches as a critical next step in frailty research.
To address this need, we propose a dynamical systems modeling approach, based on the stimulus-response experimental paradigm, to formalize and test the notion that frailty is loss of resilience. We sketch the outlines of our framework and present a “prototype” of a structural model to demonstrate that loss of resilience in homeostatic regulation can be quantified and modeled in a simple and intuitive manner as the time it takes for the system to achieve equilibrium following perturbation. We also discuss complementary modeling approaches that are applicable when there is insufficient theoretical understanding and/or data to construct structural models of the system. Our proposal is novel in that it offers a quantitative framework and methods to operationalize and test the core notion underlying frailty that it signifies a loss of resilience in homeostatic regulation.
B. Dynamical Systems Framework
A physiological system is delineated and distinguished from its surroundings by motifs such as its function (e.g. immune system), structure (e.g. mitochondria), or a chemical that is distributed in circulation (e.g. glucose). The first task is to identify the system to be studied. Then the scope of the problem, including the essential components of the system and the spatio-temporal scale of study, must be defined. We assume that these basic steps are completed. Our framework then comprises five essential elements: (1) a stimulus-response experiment or a provocative test to elicit a response from the physiological system of interest, (2) a mathematical model that describes the response, (3) estimation of model parameters using response data, (4) model criticism, and (5) systems identification.
Overall, we hypothesize that decreased resilience of homeostatic regulation in frail individuals will manifest as system responses with characteristically larger times to achieve equilibrium or reestablish homeostasis following a provocation. We describe these five elements briefly in this context and demonstrate how to model loss of resilience and to test the hypothesis that frailty is loss of resilience. We also argue, using a hypothetical physiological system example, that loss of resilience in homeostatic regulation is mainly the consequence of impaired connectivity in the regulatory networks, i.e weakened negative feedback and positive feed-forward mechanisms.
1. Stimulus-response experiment
In many areas of medicine, especially in endocrinology and cardiology, it is common to evaluate the function of a physiological system by provoking it using a chemical or physical stimulus and then monitoring its response. It has been established that appropriate provocative tests provide greater accuracy in detecting abnormalities in underlying systems than non-provocative measures. For example, the glucose and insulin responses to an oral glucose load (oral glucose tolerance test or OGTT) generally provide a more accurate characterization of impaired glucose metabolism than fasting levels of glucose and insulin [Bergman et al, 1979]. Other examples include insulin tolerance testing to evaluate the pituitary function [Fish et al, 1986], metyrapone and dexamethasone suppression tests to assess HPA axis dysfunction in patients with psychiatric disorders [Morphy, 1985], and exercise echocardiographic tests for identifying coronary artery disease in the absence of anginal symptoms [Armstrong, 1998].
2. Mathematical modeling
A dynamical systems model is a mathematical model that describes the changes in the state of a physiological system over an appropriate time interval that can range from several milliseconds (e.g. for neurons) to several days (e.g. for immune system response to vaccination). Often, models in the form of a set of differential equations depicting the mechanistic interactions between components of the system are constructed. These equations must be solved to obtain the response of the system. We term such models as “structural” models, because they posit a structure for the mechanistic interactions between system components.
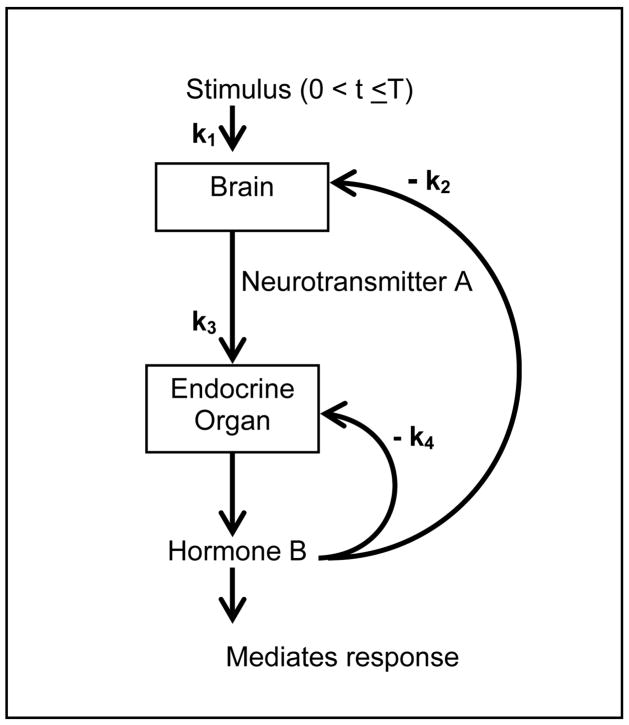
We consider a mathematical model of a hypothetical physiological system that, despite its simplicity, captures rich stimulus-response patterns postulated in the literature (Figure 1). Assume there are two biomarkers (of any closed-loop physiological system), “neurotransmitter A” and “hormone B”. Y1 is the concentration of the neurotransmitter A produced by the brain in response to a stimulus of strength k1, which then stimulates the production of a hormone B (represented by parameter k3), whose concentration is denoted by Y2. The hormone can not only inhibit its own production locally, but it can also inhibit the production of neurotransmitter via a negative feedback mechanism (represented by k2).
Figure 1.
A schematic showing the components and parameters of a hypothetical dynamical system.
The concentrations of these two biomarkers at any time t are given by Y(t) = (Y1(t), Y2(t)). The following system of differential equations depicts the interactions between the two biomarkers in our simple example:
| (1) |
where k1, k2, k3, k4 are all positive, and U{.} is an indicator function denoting application of the stimulus. Consistent with Figure 1, equation system (1) shows that the stimulus only affects Y1 with a strength of k1, and only for a finite time interval, 0 < t < T. Y1 and Y2 can be easily obtained by solving the system (1), using the standard techniques presented in texts on differential equations (see, for example, [Boyce, DiPrima, 1986], Section 7.9).
We now assume that Y2(t) can be more readily measured than Y1(t). For example, Y1 can be the concentration of CRH (corticotropin releasing hormone) and Y2 can be the concentration of ACTH. This assumption is not essential to our modeling framework, but it has been invoked to simplify the discussion of model (2). The solution for Y2 (t) is given as the sum of four exponentially decaying functions of time:
| (2) |
where: r1 = (k4 − D)/2;r2 = (k4 + D)/2; , and U(t >T) equals 1, when t > T, and is 0 otherwise. From Eq (2), we see that Y2(t) is determined by 4 parameters: k1/k2, k4, T, and D. Of these, k1/k2 only affects the scale of the response, but not the dynamics of the response, which are governed by k4, T and D.
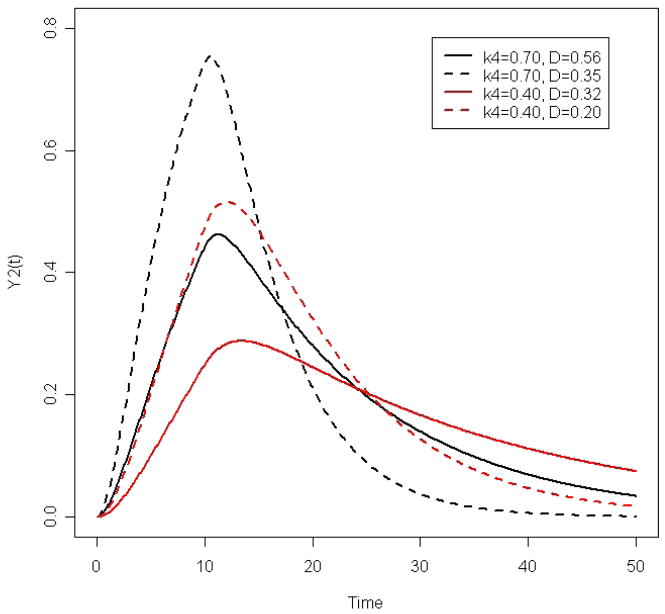
A relatively simple and intuitive measure of the resilience of a system is the time it takes for the system to achieve equilibrium following perturbation. To evaluate this, we plot Y2(t) for different values of k4 and D to understand how they affect the dynamics (Figure 2). As an example, we consider two values of k4, 0.7 and 0.4 (per time unit). For each value of k4, we consider 2 values of D such that D/k4 = 0.8 and D/k4 = 0.5, thus yielding four curves for Y2(t). We also set k1/k2 = 1 (per time unit) and T = 10 (time units). Results from these simulations are pictured in Figure 2. Increasing k4 results in faster attainment of homeostasis following stimulation (compare either the 2 solid lines or the 2 dashed lines). Increasing D results in a slower establishment of equilibrium (compare either the 2 black lines or the 2 red lines). Consequently, we hypothesize that frail individuals with decreased resilience will have smaller k4 and/or larger D. We posit that the loss of resilience of frail individuals is due to impaired negative feedback (e.g. smaller k2 and k4) and/or positive feed-forward (e.g., smaller k3) loops. Consistent with this, either smaller k2 or smaller k3 results in a larger D.
Figure 2.
Dynamic response curves of the concentration of hormone B for different values of system parameters.
3. Parameter estimation
We can characterize the dynamic response of an individual using the parameters, θ = (k1/k2, k4, D, T), which are estimated by fitting equation (2) to actual observations of Y1,obs(tj) and Y2,obs(tj), sampled at discrete times tj, using a generalized least squares estimation method [Davidian, Giltinan, 1995]. Variation of kinetic parameters defining system interactions can also be described using a two-stage hierarchical modeling strategy [Davidian, Giltinan, 1995], where the first stage model (e.g. (2)) depicts the individual response to stimulus, and the second stage model describes the joint distribution of kinetic parameters, possibly influenced by variables such as age, race, and frailty. A central hypothesis in such analysis is that the frail individuals would be characterized by a joint distribution of θ with a smaller mean of k4 and/or a larger mean of D.
4. Model Criticism
After obtaining the best fit to the data, we need to evaluate the adequacy of the model for its objectives. If there are no modeling errors, the residuals (the difference between observed and model-fitted values) should reflect experimental noise only. When the model is fitted to responses from multiple subjects, boxplots of residuals at each time of sample collection should provide a visual pattern of distribution of residuals as a function of time. Presence of a trend or other patterns in the distribution of residual would be clear signs of model inadequacy. Another check is to see whether the model can capture the variety of observed responses. For instance, the model would be inadequate if it has problems in fitting a response that does not decline but reaches a plateau. If a model is deemed inadequate, we either revise the conceptual model of the system or revise the mathematical translation of the conceptual model and repeat Steps 2 through 4.
5. Systems identification
Systems identification is aimed at characterizing how the parameters summarizing the dynamic response, θ = (k1/k2, k4, D, T), differ between frail and non-frail. The main goals are to test and elucidate the nature of differences in dynamic responses of individuals, and to evaluate whether and how these differences are related to frailty. There are two approaches to systems identification. The first, and preferred, approach is to characterize normal and abnormal responses based on a combination of physiological considerations and statistical methods, for example, cluster analysis or a latent class analysis of model parameters [Everitt, Hand, 1981]. We can then evaluate how well this characterization predicts functional manifestations of frailty such as, for example, decreased muscle strength and slow walking speed. In systems where normal and abnormal responses cannot be reliably distinguished due to a lack of physiological understanding, a second approach may be adopted. We start with some working definition of frailty (e.g. a phenotypical description), and use it to characterize how the system response to stimulus differs between frail and non-frail. This presents the difficult problem of circularity, i.e. our characterization of normal and abnormal physiological responses is not based on independent physiological consideration, but is linked to the working criterion for frailty. Hence, the characterization must be validated in independent studies for its ability to identify frail older adults.
C. Discussion
We propose an approach, based on the classical dynamical systems modeling, but demonstrating, for the first time, how it may be effectively applied to the study of loss of resilience in complex adaptive systems underlying frailty. The main novelty in our approach is that we show, using a simple prototype model (1), that loss of resilience in homeostatic regulation can be quantified and modeled in a simple and intuitive manner as the time it takes for the system to achieve equilibrium following perturbation. We hypothesize that homeostatic regulation would be compromised in frail individuals due to a breakdown in signaling pathways, i.e. a degradation of negative feedback and positive feed-forward loops. This would result in the loss of resilience characterized by a longer time to achieve homeostatic equilibrium following stimulation. Frail individuals would then be characterized by smaller kinetic parameters (in model (1)) governing the integrity of negative feedback (e.g. smaller k2 and k4) and/or positive feed-forward (e.g., smaller k3) loops. We posit that the loss of resilience of frail individuals is due to impaired negative feedback (e.g. smaller k2 and k4) and/or positive feed-forward (e.g., smaller k3) loops, and that this can be empirically tested using the proposed approach.
When structural models of a regulatory system, such as equation system (1), cannot be developed either because of insufficient theoretical understanding or due to intractable complexity of the underlying physiology, we can still obtain useful insights about the system response by adopting non-structural, statistical modeling approaches. Non-structural modeling is a more general approach to describing dynamic responses. It is commonly applied to the analysis of electrophysiological responses (e.g. ECG, EEG signals), which are typically sampled at much higher rates compared to endocrine/biochemical signals. A well-known example of this approach is the Fourier analysis of electrophysiological time series (Percival and Walden 1993). Another example of non-structural approach is the functional principal components analysis (fPCA) [Ramsay, Silverman, 2002]. fPCA breaks down the original dynamic response into constituent modes of variation over time (in this sense, fPCA is similar to, but more general than, the Fourier analysis). It can provide useful insights by identifying 1 or 2 dominant modes of dynamic responses of the physiological system, if they exist. fPCA also provides principal component (PC) scores for each individual, corresponding to each mode of variation. PC scores corresponding to the dominant modes can then be used as concise, independent summaries of the individual dynamic response.
D. Conclusion
There is emerging consensus that research on both the theory and measurement of frailty must focus on the dynamic interactions within and across the complex adaptive systems underlying the frailty syndrome. In this paper, we have proposed a testable framework, based on dynamical systems modeling, to propel future advances in that direction. The proposed dynamical systems framework offers many important benefits, including (a) insights into whether and how the response of regulatory systems putatively associated with the frailty syndrome differ between frail and non-frail older adults, (b) identification of critical regulatory systems, if they exist, that could function as sentinel systems for screening and early detection of frailty, (c) development of provocative tests that can provide maximal information on the integrity of systems identified in (b), and (d) evaluation and unification of diverse empirical descriptions of frailty by providing a mathematical framework anchored in quantifying the loss of resilience, an essential property of frailty.
Significant, but surmountable, challenges to the implementation of our paradigm include selection of physiological systems (e.g. which systems would be most relevant for the study of frailty and its functional consequences?), design of experiment (e.g. type and duration of stimulus, frequency and timing of sample collection), and feasibility of testing frail older adults due to increased burden of intensive sampling. These challenges are not insurmountable, but they need to be addressed. We hope that this paper will stimulate additional discussion on the topic, and perhaps even spur the conduct of stimulus-response experiments necessary for the next generation of research to help unravel the complex etiology of the frailty syndrome and guide translation into effective interventions to treat and ultimately, prevent frailty.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
E. References
- 1.Armstrong W, Pellikka P, Ryan T, Crouse L, Zoghbi W. Stress echocardiography: recommendations for performance and interpretation of stress echocardiography. J Amer Soc Echocardiography. 1998;11:97–104. doi: 10.1016/s0894-7317(98)70132-4. [DOI] [PubMed] [Google Scholar]
- 2.Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Chaves P, Zeger SL, Fried LP. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sc. 2006 March;61(3):262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 3.Bergman RN, Ider YZ, Bowden CR, Cobelli C. Quantitative estimation of insulin sensitivity. Am J Physiol. 1979 June;236(6):E667–677. doi: 10.1152/ajpendo.1979.236.6.E667. [DOI] [PubMed] [Google Scholar]
- 4.Bergman H, Ferrucci L, Guralnik J, Hogan DB, Hummel S, Karunananthan S, Wolfson C. Frailty: An emerging research and clinical paradigm--issues and controversies. J Gerontol A Biol Sci Med Sci. 2007 July;62(7):731–737. doi: 10.1093/gerona/62.7.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce WE, DiPrima RC. Elementary differential equations and boundary value problems. 4. New York: Wiley; 1986. [Google Scholar]
- 6.Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992 Feb;8(1):1–17. [PubMed] [Google Scholar]
- 7.Davidian M, Giltinan DM. Nonlinear models for repeated measurement data. 1. London; New York: Chapman & Hall; 1995. [Google Scholar]
- 8.Everitt BS, Hand DJ. Finite Mixture Distributions. Chapman & Hall; London: 1981. [Google Scholar]
- 9.Ferrucci L, Windham BG, Fried LP. Frailty in older persons. Genus. 2005;61:39–53. [Google Scholar]
- 10.Fish HR, Chernow B, O’Brian JT. Endocrine and neurophysiologic response of the pituitary to insulin-induced hypoglycemia: a review. Metabolism. 1986;35:763–780. doi: 10.1016/0026-0495(86)90245-3. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 March;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 12.Fried LP, Hadley EC, Walston JD, Newman AB, Guralnik JM, Studenski S, Harris TB, Ershler WB, Ferrucci L. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005 Aug 3;(31):pe24. doi: 10.1126/sageke.2005.31.pe24. 2005. [DOI] [PubMed] [Google Scholar]
- 13.Fried LP, Xue QL, Cappola AR, et al. Nonlinear, multisystem physiologic dysregulation associated with frailty in older women: implications for treatment. doi: 10.1093/gerona/glp076. (Submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leng SX, Cappola AR, Andersen RE, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16(2):153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 15.Lipsitz LA. Dynamics of stability: the physiological basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. 2002;57:115–125. doi: 10.1093/gerona/57.3.b115. [DOI] [PubMed] [Google Scholar]
- 16.Morphy MA, Fava GA, Perini GI, Molnar G, Zielezny M, Lisansky J. The dexamethasone suppression and metyrapone tests in depression. Psychiatr Res. 1985;15:153–158. doi: 10.1016/0165-1781(85)90051-4. [DOI] [PubMed] [Google Scholar]
- 17.Percival DB, Walden AT. Spectral Analysis for Physical Applications. Cambridge University Press; Cambridge, UK: 1993. [Google Scholar]
- 18.Ramsay JO, Silverman BW. Applied Functional Data Analysis. Springer; 2002. [Google Scholar]
- 19.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722–727. doi: 10.1093/gerona/62.7.722. [DOI] [PubMed] [Google Scholar]
- 20.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162(20):2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]