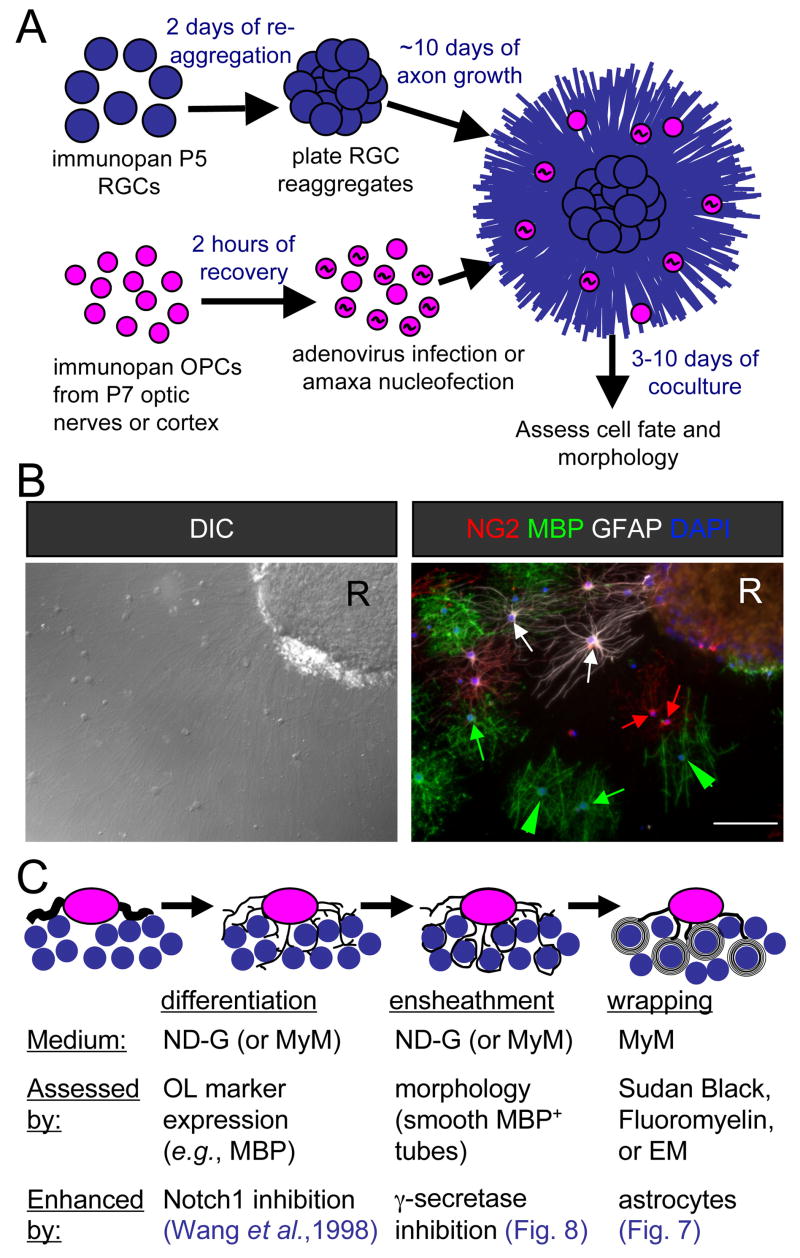
Figure 1. Schematic of myelinating cocultures of OPCs with RGC reaggregates.
(A) Perinatal (P5) RGCs are purified by immunopanning and allowed to reaggregate for 2 days on a poorly-adhesive surface prior to plating on a substrate permissive for axon growth for 7–14 days. OPCs are then acutely isolated from optic nerves or cortices and, when transfection is necessary, recovered for a couple of hours to enable recombinant adenovirus infection or amaxa nucleofection. Transfer of these OPCs to the reaggregate cultures initiates the coculture that results in myelination, analyzed by immunostaining after six days.
(B) A coculture six days after seeding of optic nerve OPCs analyzed for cell fate and morphology by differential-interference contrast (DIC) microscopy and indirect immunofluorescence. Examples of OPCs (NG2, red), non-myelinating OLs (MBP, green), and astrocytes (GFAP, white) are indicated by arrows of the corresponding colors. Myelinating OLs (green arrowheads) are identified by their extension of multiple distinctive smooth tubes. Nuclei are counterstained with DAPI (blue). An RGC reaggregate is denoted by “R”. Scale bar = 100 μm.
(C) A schematic of the development of a myelinating OL in three stages. A bipolar OPC differentiates into an immature OL that begins to express myelin proteins and extends multiple processes amongst the RGC axons. These OL processes ensheathe axons, depositing one smooth layer of membrane. The final stage consists of the wrapping of multiple layers of membrane and the extrusion of cytoplasm to form compact myelin.