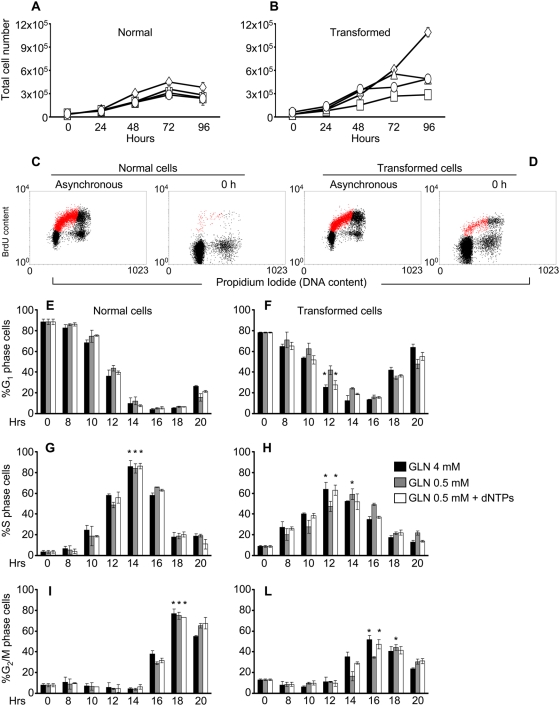
Figure 6. Proliferation analysis of normal and transformed cells adding in culture medium the four dNTPs.
Normal (A) and transformed (B) cell lines were plated as previously described. Culture medium was replaced after 24 hours (indicated as 0 h) with a normal medium −4 mM glutamine- (◊), a low medium −0.5 mM glutamine- (□), a medium with 0.5 mM glutamine and 10 µM dNTPs -ATP, GTP, TTP and CTP- (▵) and a medium with 0.5 mM glutamine and 10 µM dNTPs in which the dNTPs were added at 0 and 48 hours (○), then the cells were collected and counted at indicate time. Normal (left panels) and transformed (right panels) cells, synchronized by 24 hours serum starvation, were released in a medium containing 10% serum, 4 mM or 0.5 mM glutamine, dNTPs and BrdU for continuous DNA labeling. At indicate time points the cells were collected and stained with propidium iodide and anti-BrdU specific antibody and analyzed by flow cytometry. Representative cytograms, obtained for asynchronous and 0 hours (time 0) time point samples respectively, plotting BrdU content (labeled cells) vs. PI staining (DNA content) (Figure 6 C - normal cells - and D - transformed cells -). Red color in the plot represents the S-phase cells. The percentages of cells in G1 (panels E and F), S (panels G and H) and G2/M (panels I and L), phase of normal (left panels) and transformed (right panels) cells released in 4 mM and 0.5 mM glutamine alone or plus dNTPs obtained by bi-parametric analysis as shown above, are represented. The asterisks indicate the S or G2/M phase peaks identified in both glutamine availability. The error bars correspond to standard deviations of triplicate analysis.