Abstract
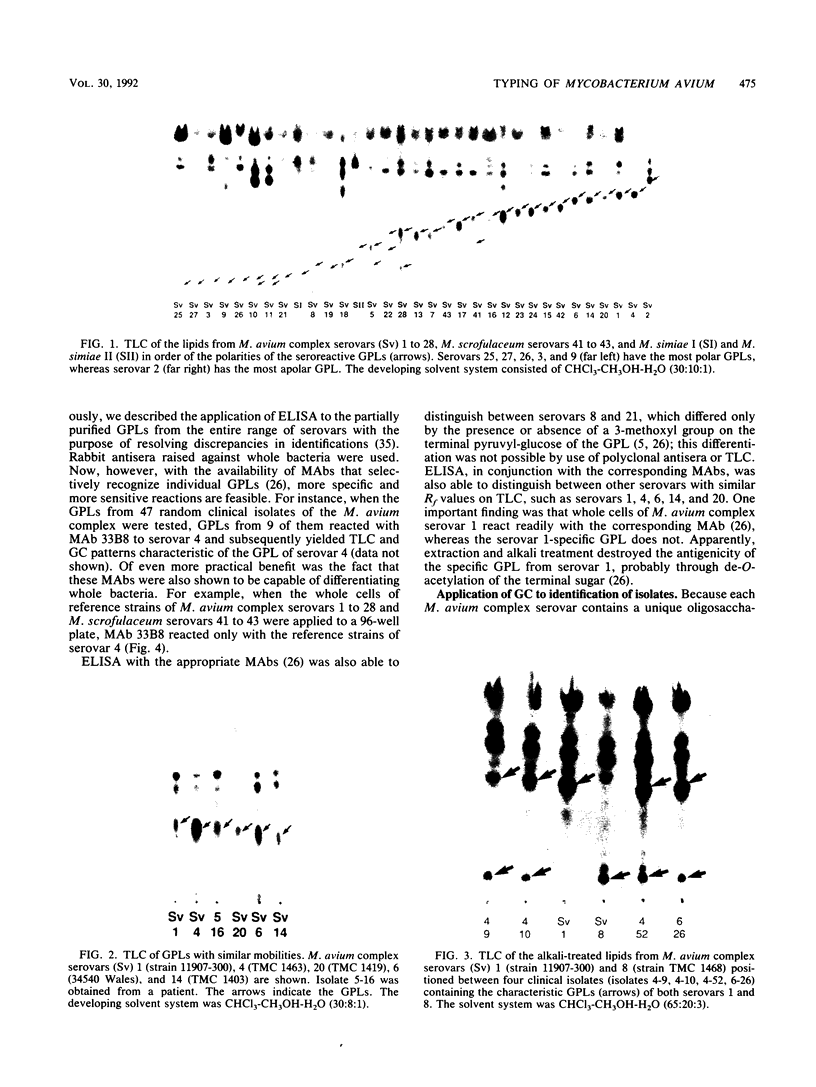
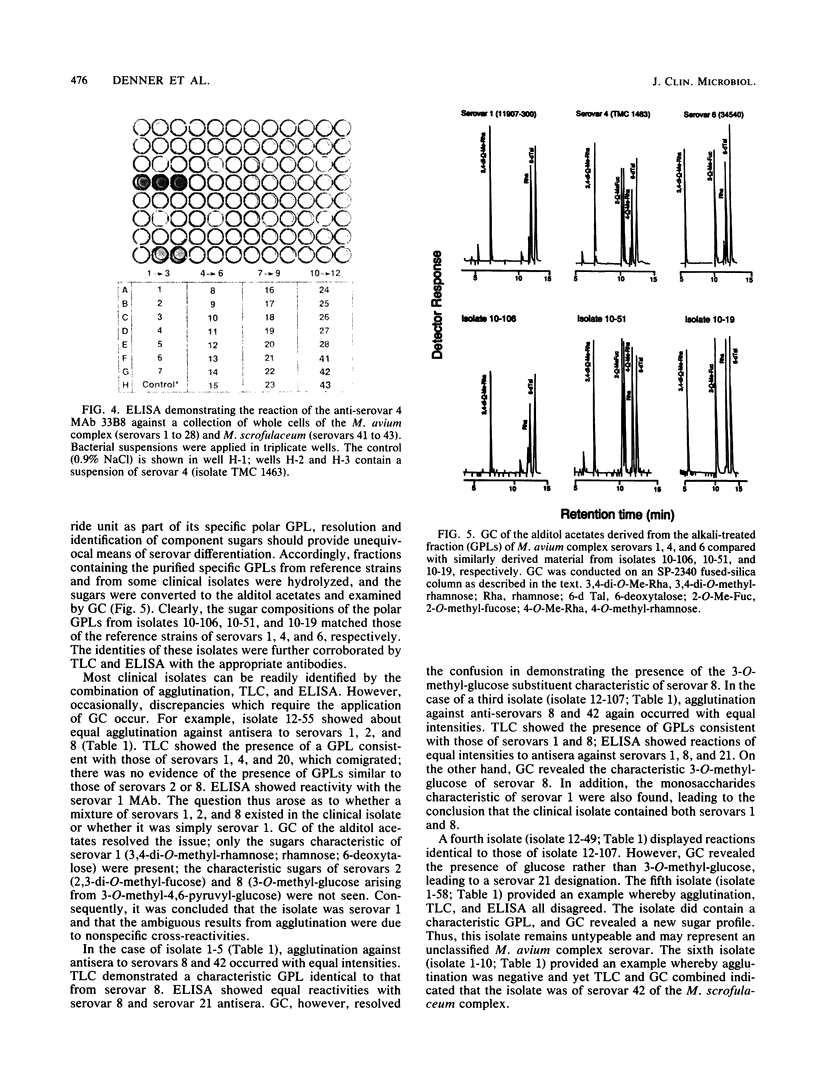
Serotyping of nontuberculous mycobacteria, especially those of the Mycobacterium avium complex, provides important epidemiological information, particularly in tracing origins of infections. Seroagglutination with whole cells and polyclonal rabbit antibodies was the original way of identifying serovars and is still commonly used. The discovery of the glycolipid nature of the typing antigens allows differentiation of serovars on the basis of thin-layer chromatography of whole antigens and gas chromatography-mass spectrometry of the characteristic sugars of the oligosaccharide haptens of these antigens. In particular, the generation of monoclonal antibodies to the glycolipid antigens allows facile differentiation of serovars through enzyme-linked immunosorbent assay. All of these protocols were applied in developing a comprehensive approach to the typing of members of the M. avium complex.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baess I. Deoxyribonucleic acid relationships between different serovars of Mycobacterium avium, Mycobacterium intracellulare and Mycobacterium scrofulaceum. Acta Pathol Microbiol Immunol Scand B. 1983 Jun;91(3):201–203. doi: 10.1111/j.1699-0463.1983.tb00033.x. [DOI] [PubMed] [Google Scholar]
- Bozic C. M., McNeil M., Chatterjee D., Jardine I., Brennan P. J. Further novel amido sugars within the glycopeptidolipid antigens of Mycobacterium avium. J Biol Chem. 1988 Oct 15;263(29):14984–14991. [PubMed] [Google Scholar]
- Brennan P. J., Aspinall G. O., Shin J. E. Structure of the specific oligosaccharides from the glycopeptidolipid antigens of serovars in the Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum complex. J Biol Chem. 1981 Jul 10;256(13):6817–6822. [PubMed] [Google Scholar]
- Brennan P. J., Goren M. B. Structural studies on the type-specific antigens and lipids of the mycobacterium avium. Mycobacterium intracellulare. Mycobacterium scrofulaceum serocomplex. Mycobacterium intracellulare serotype 9. J Biol Chem. 1979 May 25;254(10):4205–4211. [PubMed] [Google Scholar]
- Brennan P. J., Heifets M., Ullom B. P. Thin-layer chromatography of lipid antigens as a means of identifying nontuberculous mycobacteria. J Clin Microbiol. 1982 Mar;15(3):447–455. doi: 10.1128/jcm.15.3.447-455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P. J., Mayer H., Aspinall G. O., Nam Shin J. E. Structures of the glycopeptidolipid antigens from serovars in the Mycobacterium avium/Mycobacterium intracellulare/Mycobacterium scrofulaceum serocomplex. Eur J Biochem. 1981 Mar 16;115(1):7–15. doi: 10.1111/j.1432-1033.1981.tb06190.x. [DOI] [PubMed] [Google Scholar]
- Camphausen R. T., Jones R. L., Brennan P. J. Structure and relevance of the oligosaccharide hapten of Mycobacterium avium serotype 2. J Bacteriol. 1986 Nov;168(2):660–667. doi: 10.1128/jb.168.2.660-667.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D., Aspinall G. O., Brennan P. J. The presence of novel glucuronic acid-containing, type-specific glycolipid antigens within Mycobacterium spp. Revision of earlier structures. J Biol Chem. 1987 Mar 15;262(8):3528–3533. [PubMed] [Google Scholar]
- Chatterjee D., Bozic C., Aspinall G. O., Brennan P. J. Glucuronic acid- and branched sugar-containing glycolipid antigens of Mycobacterium avium. J Biol Chem. 1988 Mar 25;263(9):4092–4097. [PubMed] [Google Scholar]
- Cohn M. L., Waggoner R. F., McClatchy J. K. The 7H11 medium for the cultivation of mycobacteria. Am Rev Respir Dis. 1968 Aug;98(2):295–296. doi: 10.1164/arrd.1968.98.2.295. [DOI] [PubMed] [Google Scholar]
- Daffe M., Brennan P. J., McNeil M. Predominant structural features of the cell wall arabinogalactan of Mycobacterium tuberculosis as revealed through characterization of oligoglycosyl alditol fragments by gas chromatography/mass spectrometry and by 1H and 13C NMR analyses. J Biol Chem. 1990 Apr 25;265(12):6734–6743. [PubMed] [Google Scholar]
- Good R. C. Opportunistic pathogens in the genus Mycobacterium. Annu Rev Microbiol. 1985;39:347–369. doi: 10.1146/annurev.mi.39.100185.002023. [DOI] [PubMed] [Google Scholar]
- Guthertz L. S., Damsker B., Bottone E. J., Ford E. G., Midura T. F., Janda J. M. Mycobacterium avium and Mycobacterium intracellulare infections in patients with and without AIDS. J Infect Dis. 1989 Dec;160(6):1037–1041. doi: 10.1093/infdis/160.6.1037. [DOI] [PubMed] [Google Scholar]
- Jenkins P. A. Lipid analysis for the identification of mycobacteria: an appraisal. Rev Infect Dis. 1981 Sep-Oct;3(5):862–866. doi: 10.1093/clinids/3.5.862. [DOI] [PubMed] [Google Scholar]
- Kolk A. H., Evers R., Groothuis D. G., Gilis H., Kuijper S. Production and characterization of monoclonal antibodies against specific serotypes of Mycobacterium avium and the Mycobacterium avium-Mycobacterium intracellulare-Mycobacterium scrofulaceum complex. Infect Immun. 1989 Aug;57(8):2514–2521. doi: 10.1128/iai.57.8.2514-2521.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchy J. K. The seroagglutination test in the study of nontuberculous mycobacteria. Rev Infect Dis. 1981 Sep-Oct;3(5):867–870. doi: 10.1093/clinids/3.5.867. [DOI] [PubMed] [Google Scholar]
- McNeil M., Chatterjee D., Hunter S. W., Brennan P. J. Mycobacterial glycolipids: isolation, structures, antigenicity, and synthesis of neoantigens. Methods Enzymol. 1989;179:215–242. doi: 10.1016/0076-6879(89)79123-0. [DOI] [PubMed] [Google Scholar]
- McNeil M., Gaylord H., Brennan P. J. N-formylkansosaminyl-(1----3)-2-O-methyl-D-rhamnopyranose: the type-specific determinant of serovariant 14 of the Mycobacterium avium complex. Carbohydr Res. 1988 Jun 15;177:185–198. doi: 10.1016/0008-6215(88)85052-3. [DOI] [PubMed] [Google Scholar]
- McNeil M., Tsang A. Y., Brennan P. J. Structure and antigenicity of the specific oligosaccharide hapten from the glycopeptidolipid antigen of Mycobacterium avium serotype 4, the dominant Mycobacterium isolated from patients with acquired immune deficiency syndrome. J Biol Chem. 1987 Feb 25;262(6):2630–2635. [PubMed] [Google Scholar]
- Peterson E. M., Lu R., Floyd C., Nakasone A., Friedly G., de la Maza L. M. Direct identification of Mycobacterium tuberculosis, Mycobacterium avium, and Mycobacterium intracellulare from amplified primary cultures in BACTEC media using DNA probes. J Clin Microbiol. 1989 Jul;27(7):1543–1547. doi: 10.1128/jcm.27.7.1543-1547.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivoire B., Ranchoff B. J., Chatterjee D., Gaylord H., Tsang A. Y., Kolk A. H., Aspinall G. O., Brennan P. J. Generation of monoclonal antibodies to the specific sugar epitopes of Mycobacterium avium complex serovars. Infect Immun. 1989 Oct;57(10):3147–3158. doi: 10.1128/iai.57.10.3147-3158.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., McMillan C., Coyle M. B. Whole chromosomal DNA probes for rapid identification of Mycobacterium tuberculosis and Mycobacterium avium complex. J Clin Microbiol. 1987 Jul;25(7):1239–1243. doi: 10.1128/jcm.25.7.1239-1243.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Tomioka H., Sato K., Tasaka H., Dawson D. J. Identification of various serovar strains of Mycobacterium avium complex by using DNA probes specific for Mycobacterium avium and Mycobacterium intracellulare. J Clin Microbiol. 1990 Aug;28(8):1694–1697. doi: 10.1128/jcm.28.8.1694-1697.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer W. B. Serologic identification and classification of the atypical mycobacteria by their agglutination. Am Rev Respir Dis. 1965 Dec;92(6):85–93. doi: 10.1164/arrd.1965.92.6P2.85. [DOI] [PubMed] [Google Scholar]
- Storrs E. E. The nine-banded armadillo: a model for leprosy and other biomedical research. Int J Lepr Other Mycobact Dis. 1971 Jul-Sep;39(3):703–714. [PubMed] [Google Scholar]
- Tsang A. Y., Denner J. C., Brennan P. J., McClatchy J. K. Clinical and epidemiological importance of typing of Mycobacterium avium complex isolates. J Clin Microbiol. 1992 Feb;30(2):479–484. doi: 10.1128/jcm.30.2.479-484.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakrus M. A., Good R. C. Geographic distribution, frequency, and specimen source of Mycobacterium avium complex serotypes isolated from patients with acquired immunodeficiency syndrome. J Clin Microbiol. 1990 May;28(5):926–929. doi: 10.1128/jcm.28.5.926-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara D. L., Barr V. L., Knisley C. V., Tsang A. Y., McClatchy J. K., Brennan P. J. Enzyme-linked immunosorbent assay of glycolipid antigens for identification of mycobacteria. J Clin Microbiol. 1985 Apr;21(4):569–574. doi: 10.1128/jcm.21.4.569-574.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S. Mycobacterium avium complex infection. J Infect Dis. 1988 May;157(5):863–867. doi: 10.1093/infdis/157.5.863. [DOI] [PubMed] [Google Scholar]
- du Moulin G. C., Stottmeier K. D., Pelletier P. A., Tsang A. Y., Hedley-Whyte J. Concentration of Mycobacterium avium by hospital hot water systems. JAMA. 1988 Sep 16;260(11):1599–1601. doi: 10.1001/jama.260.11.1599. [DOI] [PubMed] [Google Scholar]