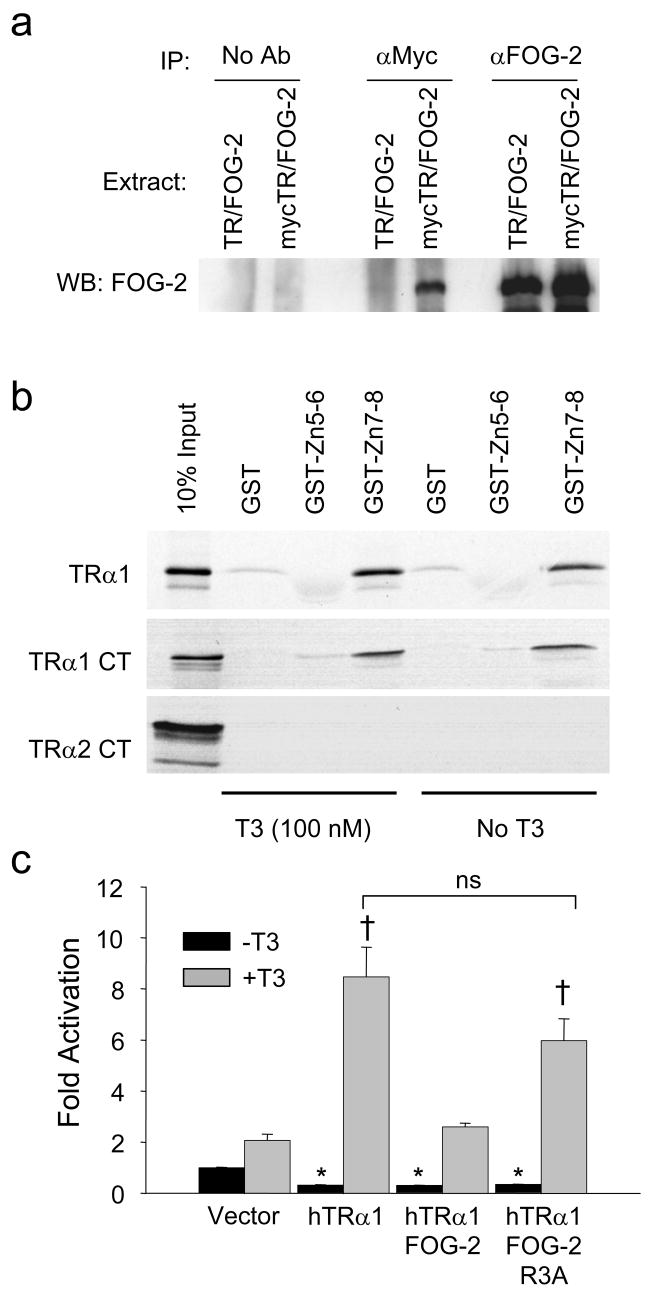
Figure 5. FOG-2 physically interacts with TRα1.
(a) Co-immunoprecipitation assay of HEK-293 cells transiently co-transfected with FOG-2 and either TRα1 or myc-tagged TRα1. Cell extracts precipitated with FOG-2 antiserum (right), myc antibody (center) and no antibody (left) were analyzed by immunoblot for FOG-2. Interaction between FOG-2 and myc-tagged TRα1 (lane 4) was specific and not a result of non-specific binding of FOG-2 to beads (lanes 1, 2) or binding of the myc-antibody to FOG-2 (lane 3). (b) GST pull-down assays of [35S] methionine-labeled full-length TRα1, TRα1-carboxy terminus only (TRα1 CT) or TRα2-carboxy terminus only (TRα2 CT) with immobilized GST-FOG-2 fusion proteins. Representative results from three experiments are shown. (c) Transient transfection assay of a TREx2-tk-luciferase reporter in cultured cardiomyocytes. FOG-2 abrogation of T3-mediated TRα1 transactivation of a plasmid is not found with the inactive FOG-2-R3A mutant. Two-way ANOVA followed by Holm-Sidak analysis, * p<0.05, versus vector control/−T3 (black bar); †, p<0.05 versus vector control/+T3 control (shaded bar).