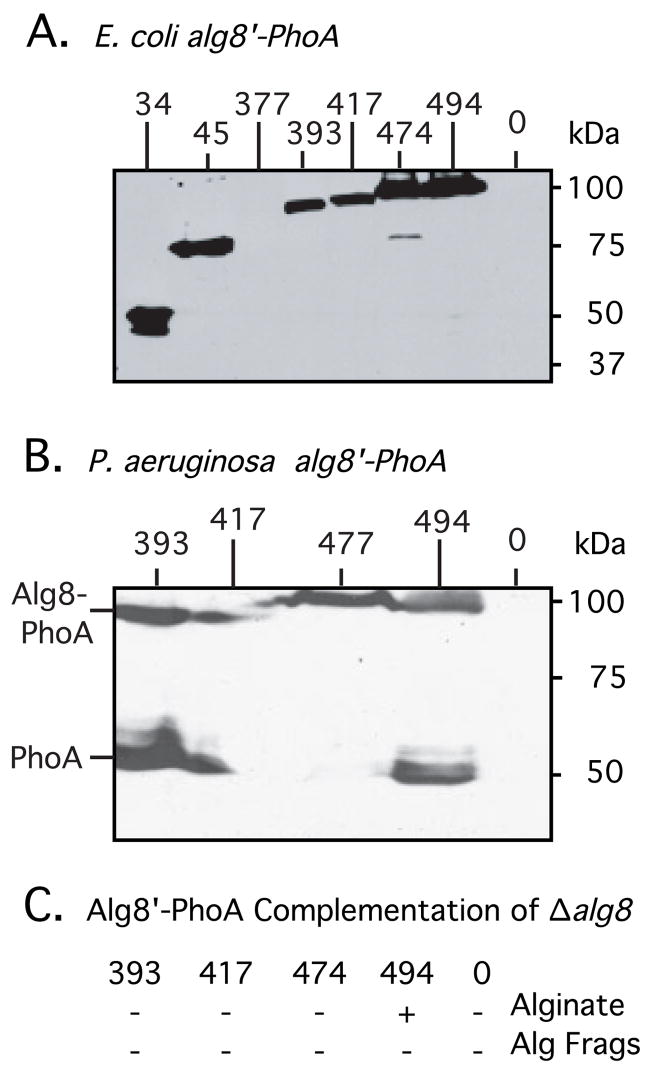
Fig. 2.
To demonstrate the expression of alg8′-phoA constructs in E. coli (A) and in P. aeruginosa FRD1108 (B), cultures were induced with IPTG, and total cell proteins were examined by Western blot analysis using anti-PhoA antibodies. Lanes are marked with the terminal amino acid in Alg8 before the PhoA fusion. Induced cultures expressed a band corresponding to the approximate size of the predicted fusion protein in E. coli. Lanes marked 0 were negative controls and contain proteins from bacteria with pLO4, which contains the unfused phoA gene cassette used to create PhoA fusions. Longer exposure was required to observe an Alg8(377)-PhoA protein of correct size encoded by pLO17, indicating that the protein was not stable. (C) Ability of Alg8-PhoA fusions, designated by their last Alg8 amino acid, to complement FRD1108 Δalg8 for alginate production or for alginate fragments (Alg Frags) is indicated.