Abstract
We expanded the meningococcal serogroup A, C, Y, and W-135 multiplex immunoassay (MIA) to simultaneously detect immunoglobulin type G antibodies directed toward Haemophilus influenzae type b polysaccharide (HibPS). The monoplex HibPS assay was compared to a HibPS-specific competitive enzyme-linked immunosorbent assay and showed a good correlation (R = 0.96). Furthermore, no cross-reactivity between HibPS and the four meningococcal serogroups was detected. This pentaplex meningococcal Hib MIA is a useful tool to investigate serological responses toward different childhood PS vaccines.
Childhood immunization programs are being expanded worldwide with various new vaccines: e.g., with Neisseria meningitidis serogroup C conjugate, pneumococcal conjugate, varicella-zoster virus, and hepatitis B virus vaccines. To reduce the number of injections, several vaccines have been developed that combine Haemophilus influenzae type b polysaccharide (HibPS), N. meningitidis serogroup C conjugate, diphtheria, tetanus, acellular pertussis, inactivated poliovirus, and/or hepatitis B virus vaccine, which in turn can be concordantly administered with other vaccines (6, 7, 9). The combination of these vaccines requires the evaluation or reevaluation of the immunogenicity of each vaccine component. Therefore, new methods have been developed, such as fluorescence multiplex immunoassays (MIAs) (1, 4, 10-13), in which the serological responses to the various vaccine antigens are determined simultaneously. MIA has the advantages of reduced laboratory time and the use of decreased amounts of specimen compared to those required by conventional methods such as enzyme-linked immunosorbent assay (ELISA).
Despite the fact that these new methods have many advantages, careful validation is needed before antibody responses toward different types of antigen (proteins or polysaccharides) can be measured in a single assay. Previously, an MIA was described in which meningococcal serogroup A, C, W-135, and Y polysaccharides were covalently attached to fluorescent beads via a poly-l-lysine (PLL) linker for the detection of specific antibody responses (1, 4). Here we describe the extension of this meningococcal MIA for the simultaneous determination of antibodies against HibPS.
Serum specimens used for evaluation of the HibPS MIA were from a study which evaluated the incidence of Bordetella pertussis infection during and shortly after pregnancy, executed during 2002 to 2006 (trial register no. ISRCTN14204141). Blood samples were obtained from mothers directly postpartum and from the umbilical cord at the time of delivery. All participants had provided informed consent at the time of enrollment to use samples anonymously for future research. From this study, a subset of serum samples (n = 75) was selected which contain HibPS-specific antibodies over a wide concentration range, induced by natural exposure to Hib.
HibPS-specific antibodies were quantified in a competitive ELISA described in detail by Mariani et al. (5), with the modification that a different secondary conjugated antibody is used: alkaline phosphatase-conjugated goat anti-human immunoglobulin G (IgG) (Sigma, St. Louis, MO). This competitive ELISA reduces the overestimation of samples in the low concentration range compared to the more conventional noncompetitive method described by Phipps et al. (8). The free HibPS competition used in this ELISA allows elimination of day-to-day background variation typical in some sera; therefore, only values representing the real anti-HibPS response are determined (5).
We used two different approaches for the detection of HibPS-specific IgG antibodies in the MIA. Initially, HibPS conjugated to methylated human serum albumin (HbO-HA; Wyeth Lederle Vaccines, Pearl River, NY), similar to the antigen used in the ELISA (5, 8), was covalently attached to fluorescent carboxylated microspheres via a carbodiimide reaction as described by Pickering et al. (11). Secondly, HibPS (Chiron, Siena, Italy) was conjugated to PLL (Sigma-Aldrich, St. Louis, MO) and subsequently to fluorescent beads according to the same procedure described for the meningococcal polysaccharides A, C, Y, and W-135 (3, 4). Subsequently, the MIA procedure for determination of total anti-HibPS IgG was performed as described for the meningococcal MIA (1, 4). Standardized reference serum lot 1983 (CBER/FDA) was used for quantitation of HibPS IgG, and standardized reference serum CDC 1992 (NIBSC, Potters Bar, United Kingdom) was used for quantitation of meningococcal serogroup A, C, Y, and W-135 IgG. For assay optimization, different types of serum diluents were used. One buffer contained 3% (wt/vol) bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO) and 0.1% (vol/vol) Tween 20 (Merck, Darmstadt, Germany) in phosphate-buffered saline (PBS), pH 7.2 (13). A second buffer consisted of 50% (vol/vol) antibody-depleted human serum (ADHS; Valley Biomedical, Winchester, VA) in PBS (2, 4).
Intra- and interassay variation, the minimal level of detection, and the lower limit of quantitation were determined as described previously (1).
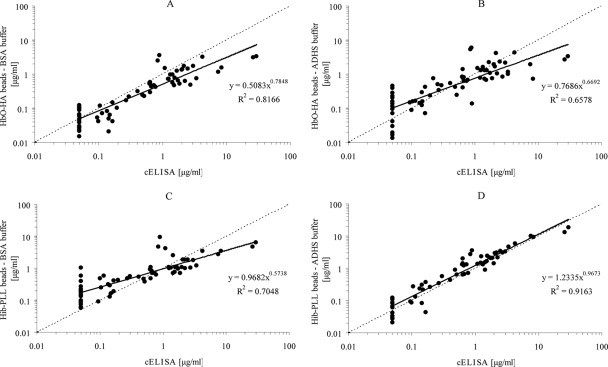
Fluorescent beads conjugated with either HbO-HA or HibPS-PLL were subsequently tested using both serum diluents and compared to the results obtained by the HibPS competitive ELISA (Fig. 1). The results clearly indicate that when HbO-HA-conjugated beads with PBS containing BSA (Fig. 1A) were used, a reduction of nonspecific binding of antibodies with a concentration of <1 μg/ml was observed, compared to the use of the ADHS buffer (Fig. 1B) or compared to the HibPS-PLL beads with the BSA buffer (Fig. 1C). The best results, however, were obtained with HibPS-PLL-conjugated beads in combination with the ADHS buffer as a serum diluent (Fig. 1D). Using these beads, in combination with the ADHS buffer, the best correlation with the HibPS competitive ELISA was observed over a wide range of antibody concentrations.
FIG. 1.
Comparison of anti-HibPS IgG levels measured by the competitive ELISA (cELISA) method with anti-HibPS IgG levels measured by the HibPS MIA. (A) ELISA comparison to HbO-HA-conjugated beads with 3% BSA serum diluent buffer. (B) ELISA comparison to HbO-HA-conjugated beads with 50% ADHS serum diluent buffer. (C) ELISA comparison to HibPS-PLL-conjugated beads with 3% BSA serum diluent buffer. (D) ELISA comparison to HibPS-PLL-conjugated beads with 50% ADHS serum diluent buffer.
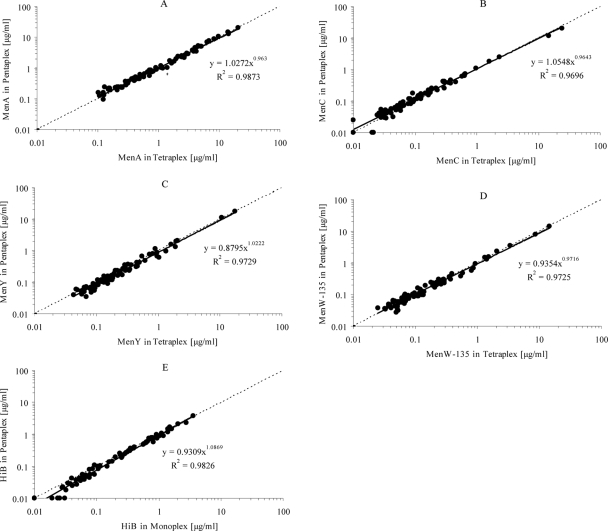
We normally perform the meningococcal MIA with the ADHS buffer as a serum diluent (4). Therefore, to expand this assay for HibPS, we used the HibPS-PLL-conjugated beads. For implementation of HibPS in the meningococcal MIA, we tested 70 serum samples from the same panel (n = 75) in the meningococcal MIA (tetraplex), the HibPS MIA (monoplex), and these two assays combined (pentaplex), to examine if no interference between beads would occur. Results were compared by linear regression, and correlation coefficients were calculated (R2 values). The individual R2 values were 0.987, 0.970, 0.973, and 0.976 for meningococcal serogroups A, C, Y, and W-135, respectively, and 0.983 for Hib (Fig. 2). We observed no interference or cross-reactivity between the meningococcal and HibPS beads. For the determination of the sensitivity for HibPS, values obtained from blank wells were used and compared to the Hib standard curve. The lower limit of quantitation was found to be 375 pg/ml anti-HibPS IgG. Intra- and interassay reproducibility was found to be high, with 5% correlation of variation and 19% correlation of variation, respectively.
FIG. 2.
Comparison of serum antibody concentrations from samples run with the meningococcal MIA (tetraplex), with the HibPS beads, and with these two assays combined (pentaplex). (A) Serogroup A (MenA). (B) Serogroup C (MenC). (C) Serogroup Y (MenY). (D) Serogroup W-135 (MenW-135). (E) Hib.
Here we describe how the meningococcal tetraplex MIA can be expanded for the simultaneous measurement of HibPS-specific IgG. Due to the use of ADHS and conjugation of HibPS to PLL, we were able to measure IgG antibodies up to more than 100-fold more sensitively than in the HibPS competitive ELISA. The HbO-HA antigen used in conventional ELISA methods can indeed also be used in MIA systems, for instance, in the assay described by van Gageldonk et al. (13). However, using HbO-HA for implementation in the meningococcal MIA, we observed a substantial amount of nonspecific binding in the low concentration range. By conjugating HibPS to PLL and using ADHS as a serum diluent, we bypass the fact that free HibPS competition is needed to overcome false-positive results. These data contribute to the growing awareness that MIAs are more sensitive and specific for measuring antibody responses than conventional ELISA methods.
Acknowledgments
We thank Pieter van Gageldonk for useful discussions and Mirte Scherpenisse for technical assistance (National Institute of Public Health and the Environment, Bilthoven, The Netherlands).
Footnotes
Published ahead of print on 7 January 2009.
REFERENCES
- 1.de Voer, R. M., F. R. M. van der Klis, C. W. A. M. Engels, G. T. Rijkers, E. A. Sanders, and G. A. M. Berbers. 2008. Development of a fluorescent-bead-based multiplex immunoassay to determine immunoglobulin G subclass responses to Neisseria meningitidis serogroup A and C polysaccharides. Clin. Vaccine Immunol. 151188-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dias, D., J. Van Doren, S. Schlottmann, S. Kelly, D. Puchalski, W. Ruiz, P. Boerckel, J. Kessler, J. M. Antonello, T. Green, M. Brown, J. Smith, N. Chirmule, E. Barr, K. U. Jansen, and M. T. Esser. 2005. Optimization and validation of a multiplexed Luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin. Diagn. Lab. Immunol. 12959-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray, B. M. 1979. ELISA methodology for polysaccharide antigens: protein coupling of polysaccharides for adsorption to plastic tubes. J. Immunol. Methods 28187-192. [DOI] [PubMed] [Google Scholar]
- 4.Lal, G., P. Balmer, H. Joseph, M. Dawson, and R. Borrow. 2004. Development and evaluation of a tetraplex flow cytometric assay for quantitation of serum antibodies to Neisseria meningitidis serogroups A, C, Y, and W-135. Clin. Diagn. Lab. Immunol. 11272-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariani, M., E. Luzzi, D. Proietti, S. Mancianti, D. Casini, P. Costantino, P. van Gageldonk, and G. Berbers. 1998. A competitive enzyme-linked immunosorbent assay for measuring the levels of serum antibody to Haemophilus influenzae type b. Clin. Diagn. Lab. Immunol. 5667-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nolan, T., S. Lambert, D. Roberton, H. Marshall, P. Richmond, C. Streeton, J. Poolman, and D. Boutriau. 2007. A novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-tetanus-toxoid conjugate vaccine is immunogenic and induces immune memory when co-administered with DTPa-HBV-IPV and conjugate pneumococcal vaccines in infants. Vaccine 258487-8499. [DOI] [PubMed] [Google Scholar]
- 7.Pace, D., M. Snape, S. Westcar, M. Hamaluba, L. M. Yu, N. Begg, J. Wysocki, H. Czajka, G. Maechler, D. Boutriau, and A. J. Pollard. 2007. A new combination Haemophilus influenzae type B and Neisseria meningitidis serogroup C-tetanus toxoid conjugate vaccine for primary immunization of infants. Pediatr. Infect. Dis. J. 261057-1059. [DOI] [PubMed] [Google Scholar]
- 8.Phipps, D. C., J. West, R. Eby, M. Koster, D. V. Madore, and S. A. Quataert. 1990. An ELISA employing a Haemophilus influenzae type b oligosaccharide-human serum albumin conjugate correlates with the radioantigen binding assay. J. Immunol. Methods 135121-128. [DOI] [PubMed] [Google Scholar]
- 9.Pichichero, M. E., H. Bernstein, M. M. Blatter, L. Schuerman, B. Cheuvart, and S. J. Holmes. 2007. Immunogenicity and safety of a combination diphtheria, tetanus toxoid, acellular pertussis, hepatitis B, and inactivated poliovirus vaccine coadministered with a 7-valent pneumococcal conjugate vaccine and a Haemophilus influenzae type b conjugate vaccine. J. Pediatr. 15143-49. [DOI] [PubMed] [Google Scholar]
- 10.Pickering, J. W., T. B. Martins, R. W. Greer, M. C. Schroder, M. E. Astill, C. M. Litwin, S. W. Hildreth, and H. R. Hill. 2002. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am. J. Clin. Pathol. 117589-596. [DOI] [PubMed] [Google Scholar]
- 11.Pickering, J. W., T. B. Martins, M. C. Schroder, and H. R. Hill. 2002. Comparison of a multiplex flow cytometric assay with enzyme-linked immunosorbent assay for quantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae type b. Clin. Diagn. Lab. Immunol. 9872-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prince, H. E., M. Lapé-Nixon, and J. Matud. 2006. Evaluation of a tetraplex microsphere assay for Bordetella pertussis antibodies. Clin. Vaccine Immunol. 13266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Gageldonk, P. G. M., F. G. van Schaijk, F. R. M. van der Klis, and G. A. M. Berbers. 2008. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J. Immunol. Methods 33579-89. [DOI] [PubMed] [Google Scholar]