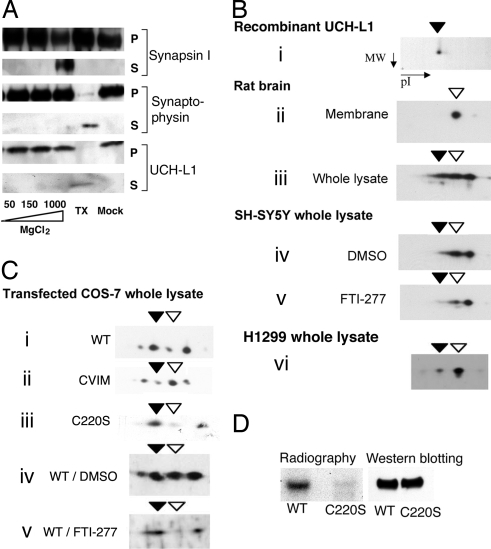
Fig. 1.
A portion of UCH-L1 is membrane-associated and farnesylated. (A) Rat brain synaptic vesicle fractions were treated with increasing salt concentrations, detergent (TX), or buffer only (Mock), as indicated. Proteins that dissociated from the vesicles were collected in supernatant fractions (S), and those that remained with the vesicular membranes were collected in the pellet fractions (P). The presence of synapsin I, synaptophysin, and UCH-L1 in each fraction was determined by Western blotting. Similar results were obtained with NaCl and KCl salts. (B) 2D electrophoresis followed by Western blot analysis using UCH-L1 antibody was performed on recombinant UCH-L1 protein (i); on the membrane fraction (ii) and whole lysate (iii) of rat brain; on lysates of SH-SY5Y cells treated with DMSO (iv) or 100 nM FTI-277 (v); and on H1299 cell lysate (vi). (C) Two-dimensional analysis was performed as in B on lysates of COS-7 cells transfected with WT (i), CVIM (ii), or C220S UCH-L1 (iii); and on lysates of WT UCH-L1-transfected cells treated with DMSO (iv) or 100 nM FTI-277 (v). Closed arrowheads indicate forms of UCH-L1 that have the same pI value as the recombinant protein; open arrowheads indicate species corresponding to the form of UCH-L1 found in the membrane fraction. (D) COS-7 cells expressing either WT or C220S FLAG-tagged UCH-L1 were radiolabeled with [3H]farnesol and immunoprecipitated by using anti-FLAG antibody. Eluted proteins were analyzed by radiography and by Western blotting for UCH-L1.