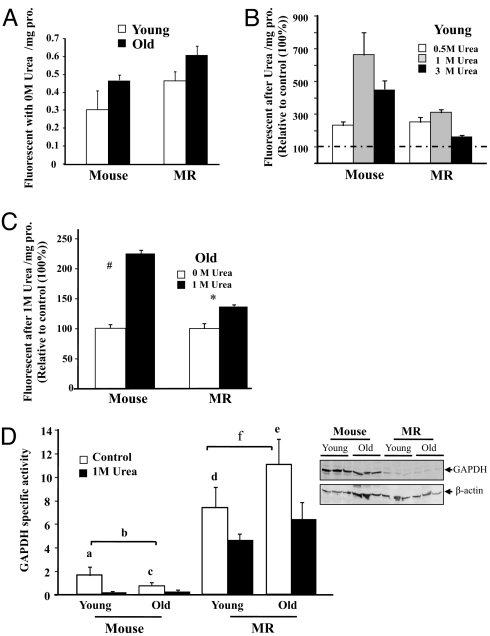
Fig. 2.
Proteins from both young and old MRs are extremely resistant to protein unfolding, whereas those from mice are markedly more susceptible. Protein unfolding is measured by incorporation of the BisANS fluorescence probe when hydrophobic pockets are exposed and is expressed as fluorescent units/mg protein as a percentage of control (100%). Data are mean ± SEM (n = 6). The # (P = 0.0038) and * (P = 0.048) denote those values that are significantly different from untreated samples when analyzed by nonparametric ANOVA. (A) Basal protein unfolding is unchanged with age (P = 0.094) in mice and MRs. (B) Young mice have markedly higher levels of protein unfolding after urea treatment than do young MRs with maximal BisANS incorporation at 1 M urea. (C) BisANS incorporation in response to1 M urea is abrogated with age, although old mice show less resistance to unfolding than do old MRs. (D) GAPDH activity declines less with urea treatment in MRs than it does in mice regardless of age, indicating that MRs are better able to maintain protein structure and function than mice. A representative Western blot of GAPDH expression is shown. Letters a–e represent significant differences among comparative datasets (a = 0.03; b = 0.05; c = 0.05; d = 0.05; e = 0.05), while f (representing age-related differences in untreated GAPDH activity in MRs) is not significant (P = 0.24).