Abstract
Tissue Doppler imaging is an echocardiographic technique that directly measures myocardial velocities. Diastolic tissue Doppler velocities reflect myocardial relaxation, and in combination with conventional Doppler measurements, ratios (transmitral early diastolic velocity/mitral annular early diastolic velocity [E/Ea]) have been developed to noninvasively estimate left ventricular (LV) filling pressure. Consequently, mitral E/Ea can help to establish the presence of clinical congestive heart failure in patients with dyspnea. However, E/Ea has a significant ‘gray zone’, and is not well validated in nonsinus rhythm and mitral valve disease. B-type natriuretic peptide (BNP) is a protein released by the ventricles in the presence of myocytic stretch, and has been correlated to LV filling pressure and, independently, to other cardiac morphological abnormalities. In addition, BNP is significantly affected by age, sex, renal function and obesity. Given its correlation with multiple cardiac variables, BNP has high sensitivity, but low specificity, for the detection of elevated LV filling pressures. Taking into account the respective strengths and limitations of BNP and mitral E/Ea, algorithms combining them can be used to more accurately estimate LV filling pressures in patients presenting with dyspnea.
Keywords: B-type natriuretic peptide, Cardiac hemodynamics, Echocardiography, Heart failure, Tissue Doppler imaging
Abstract
L’imagerie Doppler tissulaire est une technique d’échocardiographie qui mesure directement les vitesses myocardiques. Les vitesses Doppler tissulaires diastoliques sont un reflet du degré de relaxation du myocarde, et leur association à des mesures Doppler classiques a donné lieu à l’élaboration de rapports (vitesse protodiastolique transmitrale/vitesse protodiastolique annulaire mitrale [VP/VPA]) permettant d’évaluer de façon non effractive la pression de remplissage du ventricule gauche (VG). Le rapport VP/VPA mitral peut donc aider à déterminer la présence d’insuffisance cardiaque congestive clinique chez des patients dyspnéiques. Cependant, le rapport VP/VPA est associé à une grande « zone grise », et son utilisation n’est pas vraiment validée dans les cas de rythme non sinusal et de valvulopathie mitrale. De son côté, le peptide natriurétique de type B (PNB) est une protéine libérée par les ventricules lorsqu’il y a étirement des myocytes et il est corrélé avec la pression de remplissage du VG et, de façon indépendante, avec d’autres anomalies morphologiques du cœur. De plus, le PNB est grandement influencé par l’âge, le sexe, le fonctionnement rénal et l’obésité. Comme il est en corrélation avec de nombreuses variables cardiaques, le PNB a une forte sensibilité mais une faible spécificité pour la détection de pressions élevées de remplissage du VG. Compte tenu des atouts et des lacunes du PNB et du VP/VPA mitral, il est possible d’appliquer des algorithmes associant les deux mesures afin d’évaluer plus précisément qu’on le fait aujourd’hui les pressions de remplissage du VG chez des patients dyspnéiques.
Dyspnea is a common presenting symptom in patients seeking medical attention. However, determining the exact cause of dyspnea in a given patient can prove to be difficult, especially in patients with a noncardiac cause of dyspnea in the setting of known cardiac disease. Although invasively measured left ventricular (LV) filling pressure is the reference standard for establishing that pulmonary venous pressure elevation is contributing to symptoms of dyspnea in a given patient, it is not clinically feasible to subject all patients presenting with dyspnea to cardiac catheterization. Therefore, noninvasive methods of estimating LV filling pressures – B-type natriuretic peptide (BNP) measurement and the tissue Doppler (TD)-derived transmitral early diastolic/annular velocity ratio (E/Ea) – have attracted attention. Both BNP and mitral E/Ea possess important strengths and limitations. Therefore, in certain clinical scenarios, combining these indexes is superior to using either in isolation. However, there has been, to date, no clear consensus on the optimal clinical use of BNP and E/Ea in patients presenting with dyspnea. Therefore, the present paper comprehensively reviews the current literature on BNP and E/Ea, and makes clinical recommendations on the optimal use of these variables in patients with dyspnea and suspected congestive heart failure (CHF).
A BRIEF HISTORY OF TD IMAGING
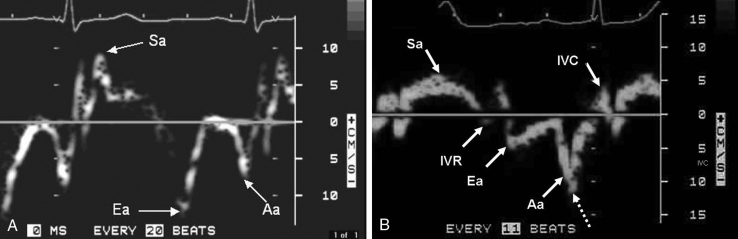
TD imaging of the heart is a Doppler technique that measures the frequency of ultrasound returning from moving myocardium to estimate the velocity of the myocardial wall. In 1989, Isaaz et al (1) were the first to describe clinical applications of TD in cardiac disease, demonstrating that low myocardial velocities at the posterior mitral annulus correlated with abnormal posterior wall motion on LV angiography. Gulati et al (2) demonstrated in 1996 that TD systolic mitral annular velocities correlated with global LV ejection fraction (EF), as assessed by radionuclide ventriculography. It was then shown that TD-derived Ea correlated with the invasively measured LV time constant of relation (Tau), establishing Ea as a relatively load-independent measure of myocardial relaxation in patients with cardiac disease (3). Using spectral TD imaging for patients 50 years of age or older, impaired myocardial relaxation is generally present when Ea is less than 10 cm/s and severely impaired when Ea is less than 6 cm/s (4). Figure 1 shows the typical appearance of TD velocities at the mitral annulus in a patient with a normal heart, and in a patient with cardiac disease but normal LVEF.
Figure 1).
A Tissue Doppler velocities in a patient with a normal heart. Both systolic annular velocity (Sa) and early diastolic annular velocity (Ea) are greater than 10 cm/s, indicating normal myocardial contractility and relaxation. B Tissue Doppler velocities in a patient with cardiac disease but normal left ventricular ejection fraction. Note that both the Sa and Ea are approximately 5 cm/s, indicating severely depressed myocardial velocities. This patient had a left ventricular ejection fraction of 64%, demonstrating myocardial disease in the presence of a normal ejection fraction. Spectral broadening (dashed arrow) can result in overestimation of tissue Doppler velocities; thus, Doppler gains must be minimized. Aa Late diastolic myocardial velocity; IVC Isovolumic contraction velocity; IVR Isovolumic relaxation velocity
TD for the assessment of diastolic function and estimation of LV filling pressures
According to Starling’s Law, in patients with normal myocardial relaxation, increases in cardiac preload are absorbed by a normally elastic LV, resulting in an increase in stroke volume and maintenance of normal left atrial (LA) pressures. However, in impaired relaxation, the LV is unable to accommodate increases in volume, resulting in an increase in LA pressure (5). Thus, given its ability to accurately measure myocardial relaxation relatively independently of LV filling pressures in patients with cardiac disease, Ea can be used to noninvasively assess LV relaxation (3). Given that Ea is virtually always depressed in patients with depressed LVEF, when transmitral E is greater than late transmitral diastolic flow (A), it can be assumed that there is elevated LV pressure (pseudonormal pattern) (6). However, with preserved LVEF, Ea is needed to determine whether the LV has normal diastolic suction, and hence, normal LA pressures (normal Ea velocity-normal filling pattern), or abnormal relaxation and elevated LA pressures (depressed Ea velocity-pseudonormal filling pattern) to determine the meaning of an E/A ratio greater than 1 in a given patient (6).
Given that peak transmitral pulsed Doppler velocity (E) varies markedly with volume, but Ea does not in patients with cardiac disease, dividing E by Ea forms an index of volume load, corrected to myocardial relaxation (3,7–10). Nagueh et al (9) first demonstrated that E/Ea correlated well (r=0.87, P<0.001) with pulmonary capillary wedge pressure using the lateral mitral annulus for the measurement of Ea. Ommen et al (10) demonstrated that E/Ea (using the mitral septal annulus) had a reasonable correlation (r=0.64, P<0.001) with mean LV diastolic pressures in patients referred for left heart catheterization (10). Mitral E/Ea has since been used in multiple patient populations to noninvasively estimate LV filling pressures in patients with cardiac disease. The main clinical uses of TD imaging are summarized in Table 1.
TABLE 1.
Summary of key information provided by tissue Doppler imaging
| Variable | Physiological meaning | Clinical use |
|---|---|---|
| Systolic annular velocity (Sa) | Sa is a more direct measure of myocardial systolic contractile function than ejection fraction alone. Sa ≥10 cm/s indicates normal contractile function, Sa <10 cm/s indicates impaired contractile function, while Sa <5 cm/s indicates severely impaired contractile function | While Sa is always depressed in the setting of abnormally depressed ejection fraction, a depressed Sa can reflect myocardial disease in the setting of normal ejection fraction (eg, hypertrophic cardiomyopathy) |
| Early diastolic annular velocity (Ea) | Ea has been correlated to the invasively measured time constant of relaxation (Tau), thus providing a measure of myocardial relaxation. An Ea ≥10 cm/s reflects normal myocardial relaxation, an Ea <10 cm/s reflects impaired myocardial relaxation and an Ea <5 cm/s reflects severely impaired myocardial relaxation | Ea distinguishes a normal transmitral filling pattern (normal relaxation with normal left ventricular filling pressures) from a pseudonormal pattern (impaired relaxation with elevated left ventricular filling pressures), because both of these are seen as transmitral early diastolic velocity > late diastolic velocity (E>A) on the transmitral filling profile |
| Transmitral early diastolic velocity/tissue Doppler Ea (E/Ea) | Dividing E by Ea results in a ratio (E/Ea) that reflects left ventricular filling pressure. It should be noted that in patients with completely normal hearts (ie, normal structure, function and tissue Doppler indexes), the E/Ea is not a reliable measure of left ventricular filling pressure | An E/Ea ≥10 indicates normal left ventricular filling pressures, while an E/Ea ≥15 indicates elevated left ventricular filling pressures. An E/Ea from 11 to 14 is a gray zone, in which case other variables are needed to determine whether left ventricular filling pressures are elevated (see Table 2) |
Limitations of the use of mitral E/Ea for the estimation of LV filling pressure and as an aid in the diagnosis of clinical CHF
The accuracy of E/Ea for estimating LV filling pressure appears to be better in patients with depressed LVEF (less than 50%) than in patients with preserved LVEF (50% or greater). In a study of 71 patients undergoing cardiac catheterization (11), E/Ea had a significant correlation (r=0.80) with LV filling pressure in patients with LVEF less than 50% compared with a significant, but lower, correlation of 0.57 in patients with LVEF 50% or greater. There have also been varying data on which mitral annulus to use for the measurement of Ea in the apical four-chamber view. Given that the septal myocardial velocities are generally lower than the lateral velocities, an E/Ea of 12 or greater at the lateral mitral annulus and an E/Ea of 15 or greater at the septal annulus reflect elevated LV filling pressures (9–11). Particularly when regional wall motion abnormalities are present, the laboratory at the Baylor College of Medicine (Houston, USA) generally averages the Ea from the septal and lateral mitral annuli, with an E/Ea of 15 or greater reflecting elevated LV filling pressures (12).
E/Ea has not been validated in patients with severe mitral annular calcification, mitral stenosis or a prosthetic mitral valve. In the setting of significant mitral regurgitation, initial reports have indicated that E/Ea can be used to estimate LV filling pressure (13). In addition, in patients with paced rhythm, mitral E/Ea can be difficult to measure and has not been validated for LV filling pressure estimation. In patients with atrial fibrillation, one group (14) has demonstrated that mitral E/Ea correlates (r=0.79) with invasively measured LV filling pressure (P<0.001), assuming that velocities from 10 cardiac cycles are averaged. However, in atrial fibrillation, we find that shortened mitral E acceleration and deceleration times are as valuable as E/Ea in predicting elevated filling LV filling pressures (15).
Importantly, there is evidence suggesting a ‘gray zone’ when using E/Ea for the estimation of LV filling pressures. In general, an an E/Ea of 15 or greater suggests a pulmonary capillary wedge pressure of 15 mmHg or more, while an E/Ea of 10 or less suggests a pulmonary capillary wedge pressure of less than 15 mmHg (9). However, for patients with an E/Ea from 11 to 14, it can be difficult to accurately estimate LV filling pressures using E/Ea alone. In such a scenario, a normal LA volume index (less than 32 mL/m2) is relatively specific for normal LV filling pressures (16). In addition, given that the gray zone of the mitral E/Ea can be frequently encountered in clinical practice, BNP can be added to the equation to more accurately rule in or rule out LV filling pressure elevation. The limitations of using TD in patients to assess diastolic function are summarized in Table 2.
TABLE 2.
Important limitations of tissue Doppler variables
| Variable | Limitations |
|---|---|
| Doppler angulation error | The vector of the Doppler sample volume must deviate <20° from vector of myocardial motion to avoid significant underestimation of myocardial velocities |
| Which annulus: septal or lateral? | Given that early diastolic annular velocity (Ea) at the septal annulus is lower than Ea at the lateral annulus, the early diastolic velocity to Ea (E/Ea) ratio at the septal annulus is almost always higher than E/Ea at the lateral annulus. Ea at both annuli can be averaged to calculate E/Ea, especially in patients with regional wall motion abnormalities. Thus, E/Ea (septal) ≥15, E/Ea (lateral) ≥12 and E/Ea (average) ≥15 can be used to reflect elevated left ventricular filling pressures |
| Preserved ejection fraction (≥50%) versus normal hearts | In general, E/Ea is more accurate in estimating left ventricular filling pressures in patients with depressed, rather than preserved, ejection fraction |
| E/Ea ‘gray zone’ | An E/Ea between 11 and 14 is a gray zone, in which case other variables are needed to accurately estimate left ventricular filling pressure: left atrial volume indexed to body surface area (a normal volume, <32 mL/m2, effectively rules out significant left atrial pressure elevation), pulmonary venous flow profile (systolic wave > diastolic wave, implying normal left ventricular filling pressures) and B-type natriuretic peptide (a level <50 pg/mL effectively excluding elevated left ventricular filling pressure) |
| Clinical situations in which E/Ea has not been well validated | The E/Ea is not generally used in patients with severe mitral annular calcification, mitral stenosis and mitral prosthetic valves. In patients with hemodynamically significant mitral regurgitation and in patients with atrial fibrillation, caution must be used when using E/Ea (see text). The E/Ea has not been validated in paced rhythm |
BNP for the estimation of LV filling pressures
Given that BNP is a protein released from the cardiac ventricles in response to myocytic stretch, elevated LV diastolic pressures cause elevation of plasma BNP (17). Although a significant correlation (r=0.72) has been shown between BNP and pulmonary capillary wedge pressure in patients with depressed LVEF and CHF (18), there are important limitations to consider. In patients with advanced systolic CHF admitted to hospital who had markedly elevated wedge pressure and marked elevation of BNP, after intravenous diuresis and afterload reduction, wedge pressure returned to normal (less than 15 mmHg), but BNP remained markedly elevated (18). Thus, the underlying cardiac structural derangements that were unchanged by diuresis caused persistent elevation in BNP levels, despite normalization of wedge pressure. In another study of 72 patients with symptomatic LV dysfunction (19), BNP had a significant but modest correlation (r=0.57) with LV end diastolic pressure. In critical care patients with indwelling pulmonary artery catheters, our group demonstrated (20) a weak correlation (r=0.32) between BNP level and pulmonary capillary wedge pressure. In a study by Forfia et al (21) measuring peptide levels in a heterogeneous group of 40 critical care patients who had invasive measurement of pulmonary capillary wedge pressure, concentrations of BNP (median 420 pg/mL) and N-terminal proBNP (NT-proBNP) (median 3304 pg/mL) were markedly elevated; however, both BNP (r=0.40) and NT-proBNP (r=0.32) had weak correlations with pulmonary capillary wedge pressure.
BNP as an aid in the clinical diagnosis of CHF
BNP has been also used to detect CHF in outpatients with dyspnea (22) and clinical CHF in patients presenting emergently with dyspnea (23). In a multicentre trial of 1586 patients presenting to the emergency department with acute dyspnea (23), BNP levels greater than 80 pg/mL had a sensitivity of 93% and a specificity of 74% for identifying clinical CHF based on the Framingham criteria. Higher cut-off values of BNP had higher specificity, but lower sensitivity for the diagnosis of CHF, while lower BNP cut-off values had higher sensitivity but lower specificity for CHF. In general, the ‘sicker’ the patient population, and the higher the prevalence of cardiac disease, the higher the BNP cut-off is needed to rule in (or out) CHF as the cause of dyspnea. For instance, in 163 patients admitted to hospital for suspected CHF, the best cutoff value of BNP for the clinical diagnosis of CHF was greater than 300 pg/mL, with a sensitivity of 88% and a specificity of 87%; to rule out CHF with 93% accuracy, a BNP level greater than 80 pg/mL was needed (24).
BNP versus NT-proBNP
NT-proBNP is the amino terminal fragment of the BNP molecule, and has also been used to reflect elevated LV filling pressures and support the diagnosis of CHF in patients presenting with dyspnea. In a pooled analysis of 1256 patients with dyspnea (25), an NT-proBNP level of less than 300 pg/mL had a 98% negative predictive value to exclude acute CHF (with a specificity of 60%). Similar to the findings for BNP, Joung et al (26) compared invasively measured LV filling pressures to NT-proBNP levels in 214 patients referred for diagnostic angiography, finding that there were significant, but weak, correlations of NT-proBNP levels with LV end-diastolic pressure (r=0.33) and LV preatrial contraction pressure (r=0.31).
Although both natriuretic peptides are affected by renal function, NT-proBNP may be more significantly affected by renal impairment than BNP. In the study by Forfia et al (21), both natriuretic peptide levels were four times greater in patients with impaired (estimated glomerular filtration rate of less than 60 mL/min/1.73 m2) versus normal renal function, despite similar wedge pressure, cardiac index and LVEF. In addition, both BNP and NT-proBNP showed stronger correlations with pulmonary capillary wedge pressure in patients with preserved (BNP r=0.58, NT-proBNP r=0.73) compared with impaired renal function (BNP r=0.48, NT-proBNP r=0.34). However, overall, studies of NT-proBNP show relative comparability with BNP, although different cut-off values are necessary to rule in or rule out elevated LV filling pressures.
Limitations of BNP for the estimation of LV filling pressure and as an aid in the diagnosis of clinical CHF
Because multiple cardiac factors, independent of LV filling pressure, can cause BNP elevations (depressed LVEF, increased LV mass, depressed right ventricular [RV] function, elevated pulmonary artery pressure, significant mitral regurgitation and significant aortic stenosis), BNP elevation alone does not point to elevation of LV filling pressures per se, but to a significant functional or morphological cardiac abnormality (20,26–31). In addition, severe systemic syndromes, such as sepsis, can produce elevated BNP levels in the absence of CHF. A recent study (32) demonstrated that in 249 critically ill patients, patients with CHF confirmed by invasive hemodynamic measurements had BNP and NT-proBNP levels comparable with those of patients with sepsis, who did not have invasive hemodynamic evidence of CHF.
Studies (33–34) have also shown that significant BNP elevations can occur in acute pulmonary embolism without any evidence of LV heart failure. In addition, elevation of BNP has prognostic importance in pulmonary embolism; in one study (33), a BNP level of less than 21.7 pg/mmol excluded fatal pulmonary embolism with 99% accuracy, while a second study (34) demonstrated that a BNP level of less than 50 pg/mmol excluded an adverse outcome from acute pulmonary embolism with 95% accuracy. Similar findings were made with NT-proBNP levels in patients with acute pulmonary embolism (35). Therefore, being released by RV dysfunction in the absence of LV dysfunction, BNP elevations do not always suggest the presence of LV failure.
In patients with atrial fibrillation, there is conflicting evidence on the use of BNP to reflect LV filling pressure elevation. Some studies have demonstrated that atrial fibrillation can cause elevated BNP levels (36), whereas others have demonstrated that atrial fibrillation in isolation does not cause BNP elevation (37). Another potential limitation to the use of BNP is in patients who are obese (body mass index of 30 kg/m2 or greater). Various studies (38–42) have demonstrated that BNP (and NT-proBNP) levels are lower in obese patients, with and without heart failure, than in nonobese comparators. The mechanisms behind lower circulating BNP levels in obese patients are unclear, but may include decreased production combined with increased clearance of BNP. Nevertheless, some investigators have suggested significantly lower BNP cut-off values in obese patients to indicate the presence of CHF, although there has been no consensus on which levels are appropriate (43).
BNP and NT-proBNP levels also appear to also be affected by sex and age. In a cohort of normal patients identified from the Olmsted county database (44), BNP measured using the commonly used Biosite assay (Biosite Inc, USA) was 80% greater in women than in men, and increased progressively with age (by roughly 10 pg/mL per decade). Similarly, data on normal patients from the same population revealed that NT-proBNP levels were approximately three times greater in women than men, also increasing by approximately 10 pg/mL to 20 pg/mL per decade (45).
Overall, because BNP is elevated by multiple cardiac abnormalities, independent of LV filling pressure, BNP has a very high sensitivity, but low specificity, for elevated LV filling pressures. In addition, important patient factors such as age, sex, renal function and obesity affect BNP (and NT-proBNP) levels. Given such limitations, there has been interest in combining BNP with the E/Ea to more accurately assess LV filling pressures.
Combining BNP and TD in the assessment of LV filling pressures
There are relatively few data comparing the accuracy of using BNP or the E/Ea alone, or in combination, for the diagnosis of CHF. However, given the limitations associated with using either variable alone, there is interest in combining BNP and the E/Ea to aid in the diagnosis of CHF. In 106 patients with symptomatic systolic heart failure (LVEF less than 35%), BNP had a significant, but modest, correlation (r=0.51) with the mitral E/Ea, because it significantly and independently correlated with LVEF, RV systolic dysfunction, mitral regurgitation grade, age and creatinine clearance (31). In 122 patients hospitalized with dyspnea and suspected CHF (46), we demonstrated that BNP 250 pg/mL or greater and a mitral of E/Ea 15 or greater were comparably accurate (80%) in diagnosing clinical CHF using the Framingham criteria. In 78 patients with confirmed diastolic heart failure (LVEF 45% or greater) compared with 38 patients with noncardiac dyspnea, BNP greater than 146 pg/mL (sensitivity of 90% and specificity of 76%) and the E/Ea greater than 11.5 (sensitivity of 80% and specificity of 94%) were similarly accurate for predicting decompensated CHF, and the E/Ea added independent additional information to clinical judgment and BNP (47). In 108 patients referred for echocardiography (48), although BNP levels correlated to E/Ea, using either index alone was inferior to combining them for the estimation of LV filling pressure.
In unselected critical care patients with indwelling pulmonary artery catheters, the E/Ea greater than 15 and BNP greater than 300 pg/mL have been shown to be highly and comparably sensitive for pulmonary capillary wedge pressure of greater than 15 mmHg, but the E/Ea was more specific because it is not elevated by cardiac structural abnormalities alone (independent of LV filling pressure) (20). However, in patients with structurally normal hearts (such as young trauma patients), BNP was shown to correlate better than E/Ea with pulmonary capillary wedge pressure, because Ea varies significantly with volume load in normal hearts.
Therefore, because BNP is elevated by multiple cardiac conditions in addition to elevated filling pressures, it can be seen as a marker of cardiac disease in general, leaving open a window for the addition of the TD-derived mitral E/Ea, because the latter is more specific for LV filling pressures. While BNP has an excellent negative predictive value for the presence of CHF (levels greater than 50 pg/mL in patients presenting to the emergency department with acute dyspnea effectively excluding acute CHF [23]), elevated levels need to be followed by determination of the E/Ea to specifically assess LV diastolic function. In addition, a comprehensive echocardiographic Doppler study (6) also identified causes of elevated BNP independent of elevated LV filling pressures, such as depressed LV and RV function, dilated heart chambers, significant valve disease, pericardial disease and pulmonary hypertension. The pros and cons of using TD and BNP to estimate LV filling pressures are summarized in Table 3.
TABLE 3.
Pros and cons of tissue Doppler-derived early diastolic velocity to early diastolic annular velocity ratio (E/Ea) and B-type natriuretic peptide for the estimation of left ventricular filling pressures
| B-type natriuretic peptide | E/Ea |
|---|---|
| Pros | |
| Point-of-care | High specificity |
| Inexpensive | Accompanying echocardiographic Doppler information, is very useful to |
| Rapid result | determine cause of dyspnea |
| Excellent negative predictive value | Can be used in obese patients |
| Well validated for clinical congestive heart failure, less so with invasive hemodynamic indexes | Rapidly changes in response to volume changes Validated in multiple studies and patient populations |
| Cons | |
| ‘Gray zone’ | ‘Gray zone’ |
| Low specificity | In general, not point-of-care |
| Renal failure can create false-positive results | More expensive |
| Unclear use in obese patients | Can be unclear in patients with poor echocardiographic windows |
| Cannot distinguish among causes of heart failure (valvular, diastolic, restrictive, right ventricular, etc) | Not well validated in mitral valve disease (stenosis, regurgitation, prosthesis) |
| Not well validated in nonsinus rhythm | |
| Unclear use in atrial fibrillation | |
| B-type natriuretic peptide ‘memory’ – may not respond rapidly to volume changes | |
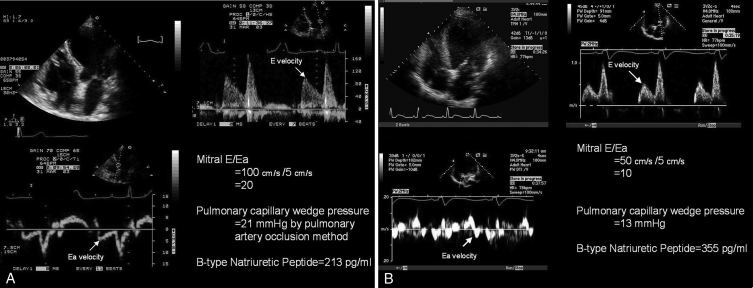
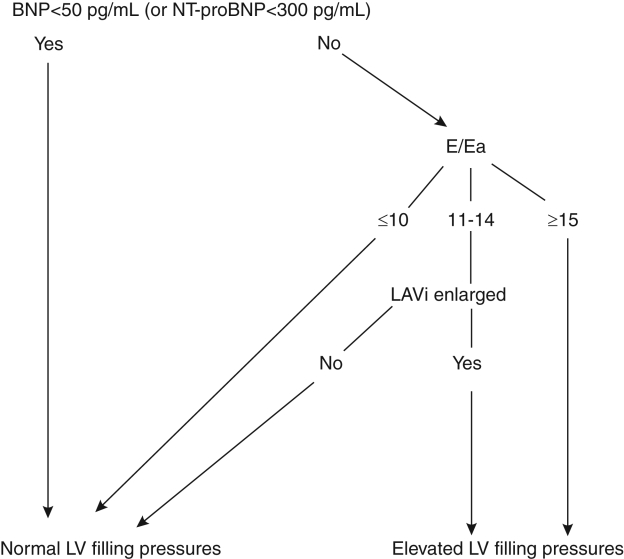
Figure 2A demonstrates a case of heart failure with a preserved LVEF and elevated BNP, while Figure 2B illustrates that patients with depressed LVEF but normal filling pressures have normal E/Ea, but elevated BNP levels. Thus, using BNP alone in this latter scenario may falsely result in a diagnosis of clinical CHF in a patient with well-compensated LV filling pressures but with LV systolic dysfunction. Figure 3 provides an algorithm for combining BNP (or NT-proBNP) and the E/Ea to assess LV filling pressures in patients presenting with dyspnea.
Figure 2).
A Tissue Doppler imaging in the prediction of left ventricular (LV) filling pressures: normal LV ejection fraction. This patient had an LV ejection fraction of 67% and clinical congestive heart failure. The mitral early diastolic pulsed Doppler transmitral inflow velocity to early diastolic annular velocity ratio (E/Ea) was 20, indicating elevated LV filling pressures. Simultaneous pulmonary capillary wedge pressure by Swan-Ganz catheterization was 21 mmHg. Given the elevated filling pressures, in the presence of preserved ejection fraction, the B-type natriuretic peptide level was elevated (213 pg/mL). Thus, this patient had heart failure with a normal ejection fraction. B Tissue Doppler imaging in the prediction of LV filling pressures: depressed LV ejection fraction. This patient had known depressed LV function with an ejection fraction of 29%, but did not have clinical congestive heart failure. The mitral E/Ea ratio was 10, indicating normal LV filling pressures. Simultaneous pulmonary capillary wedge pressure by Swan-Ganz catheterization was 13 mmHg. Despite the normal filling pressures, the B-type natriuretic peptide level was elevated (355 pg/mL), owing to LV dilation and depressed ejection fraction
Figure 3).
Using B-type natriuretic peptide (BNP) and the early diastolic velocity to early diastolic annular velocity ratio (E/Ea) to predict left ventricular (LV) filling pressures in patients presenting with acute dyspnea. Left atrial volume indexed to body surface area (LAVi) ≥32 mL/m2 is enlarged and LAVi <32 mL/m2 is normal. NT-proBNP N-terminal proBNP
CONCLUSIONS
TD imaging directly measures myocardial velocities and is an important variable in assessing LV diastolic function. The TD-derived E/Ea is being used to noninvasively estimate LV filling pressures in a widening group of patients with dyspnea and suspected CHF. BNP (and NT-proBNP) are also valuable aids in diagnosing CHF in patients with dyspnea. However, because both the E/Ea and BNP have important limitations, there is interest in defining their complementary roles in the clinical setting. In general, E/Ea is generally more specific than BNP for elevated filling pressures because BNP is elevated by cardiac structural abnormalities alone. However, BNP has a high negative predictive value for excluding CHF. Therefore, an algorithm combining BNP and the E/Ea can be used to estimate LV filling pressures in patients presenting with acute dyspnea and suspected CHF.
REFERENCES
- 1.Isaaz K, Thompson A, Ethevenot G, Cloez JL, Brembilla B, Pernot C. Doppler echocardiographic measurement of low velocity motion of the left ventricular posterior wall. Am J Cardiol. 1989;64:66–75. doi: 10.1016/0002-9149(89)90655-3. [DOI] [PubMed] [Google Scholar]
- 2.Gulati VK, Katz WE, Follansbee WP, Gorcsan J., III Mitral annular descent velocity by tissue Doppler echocardiography as an index of global left ventricular function. Am J Cardiol. 1996;77:979–84. doi: 10.1016/s0002-9149(96)00033-1. [DOI] [PubMed] [Google Scholar]
- 3.Sohn DW, Chai IH, Lee DJ, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–80. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 4.Alam M, Wardell J, Andersson E, Samad BA, Nordlander R. Characteristics of mitral and tricuspid annular velocities determined by pulsed wave Doppler tissue imaging in healthy subjects. J Am Soc Echocardiogr. 1999;12:618–28. doi: 10.1053/je.1999.v12.a99246. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosetta Stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 6.Oh JK, Hatle L, Tajik AJ, Little WC. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol. 2006;47:500–6. doi: 10.1016/j.jacc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Nishimura RA, Chaliki HP, Appleton CP, Holmes DR, Jr, Redfield MM. Determination of left ventricular filling pressure by Doppler echocardiography in patients with coronary artery disease: Critical role of left ventricular systolic function. J Am Coll Cardiol. 1997;30:1819–26. doi: 10.1016/s0735-1097(97)00390-2. [DOI] [PubMed] [Google Scholar]
- 8.Firstenberg MS, Levine BD, Garcia MJ, et al. Relationship of echocardiographic indices to pulmonary capillary wedge pressures in healthy volunteers. J Am Coll Cardiol. 2000;36:1664–9. doi: 10.1016/s0735-1097(00)00909-8. [DOI] [PubMed] [Google Scholar]
- 9.Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: A noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–33. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 10.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–94. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 11.Kidawa M, Coignard L, Drobinski G, et al. Comparative value of tissue Doppler imaging and m-mode color Doppler mitral flow propagation velocity for the evaluation of left ventricular filling pressure. Chest. 2005;128:2544–50. doi: 10.1378/chest.128.4.2544. [DOI] [PubMed] [Google Scholar]
- 12.Rivas-Gotz C, Manolios M, Thohan V, Nagueh SF. Impact of left ventricular ejection fraction on estimation of left ventricular filling pressures using tissue Doppler and flow propagation velocity. Am J Cardiol. 2003;91:780–4. doi: 10.1016/s0002-9149(02)03433-1. [DOI] [PubMed] [Google Scholar]
- 13.Bruch C, Stypmann J, Gradaus R, Breithardt G, Wichter T. Usefulness of tissue Doppler imaging for estimation of filling pressures in patients with primary or secondary pure mitral regurgitation. Am J Cardiol. 2004;93:324–8. doi: 10.1016/j.amjcard.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Sohn DW, Song JM, Zo JH, et al. Mitral annulus velocity in the evaluation of left ventricular diastolic function in atrial fibrillation. J Am Soc Echocardiogr. 1999;12:927–31. doi: 10.1016/s0894-7317(99)70145-8. [DOI] [PubMed] [Google Scholar]
- 15.Nagueh SF, Kopelen HA, Quiñones MA. Assessment of left ventricular filling pressures by Doppler in the presence of atrial fibrillation. Circulation. 1996;94:2138–45. doi: 10.1161/01.cir.94.9.2138. [DOI] [PubMed] [Google Scholar]
- 16.Abhayaratna WP, Seward JB, Appleton CP, et al. Left atrial size: Physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–63. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 17.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316–22. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- 18.Kazanegra R, Cheng V, Garcia A, et al. A rapid test for B-type natriuretic peptide correlates with falling wedge pressures in patients treated for decompensated heart failure: A pilot study. J Card Fail. 2001;7:21–9. doi: 10.1054/jcaf.2001.23355. [DOI] [PubMed] [Google Scholar]
- 19.Maeda K, Tsutamoto T, Wada A, Hisanaga T, Kinoshita M. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135:825–32. doi: 10.1016/s0002-8703(98)70041-9. [DOI] [PubMed] [Google Scholar]
- 20.Dokainish H, Zoghbi WA, Lakkis NM, et al. Optimal noninvasive assessment of left ventricular filling pressures: A comparison of tissue Doppler echocardiography and B-type natriuretic peptide in patients with pulmonary artery catheters. Circulation. 2004;109:2432–9. doi: 10.1161/01.CIR.0000127882.58426.7A. [DOI] [PubMed] [Google Scholar]
- 21.Forfia PR, Watkins SP, Rame JE, Stewart KJ, Shapiro EP. Relationship between B-type natriuretic peptides and pulmonary capillary wedge pressure in the intensive care unit. J Am Coll Cardiol. 2005;45:1667–71. doi: 10.1016/j.jacc.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 22.Cowie MR, Struthers AD, Wood DA, et al. Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet. 1997;350:1349–53. doi: 10.1016/S0140-6736(97)06031-5. [DOI] [PubMed] [Google Scholar]
- 23.Maisel AS, Krishnaswamy P, Nowak RM, et al. Breathing Not Properly Multinational Study Investigators Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–7. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 24.Logeart D, Thabut G, Jourdain P, et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol. 2004;43:635–41. doi: 10.1016/j.jacc.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 25.Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: An international pooled analysis of 1256 patients: The International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27:330–7. doi: 10.1093/eurheartj/ehi631. [DOI] [PubMed] [Google Scholar]
- 26.Joung B, Ha JW, Ko YG, et al. Can pro-brain natriuretic peptide be used as a noninvasive predictor of elevated left ventricular diastolic pressures in patients with normal systolic function? Am Heart J. 2005;150:1213–9. doi: 10.1016/j.ahj.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 27.McDonagh TA, Robb SD, Murdoch DR, et al. Biochemical detection of left-ventricular systolic dysfunction. Lancet. 1998;351:9–13. doi: 10.1016/s0140-6736(97)03034-1. [DOI] [PubMed] [Google Scholar]
- 28.Leuchte HH, Holzapfel M, Baumgartner RA, et al. Clinical significance of brain natriuretic peptide in primary pulmonary hypertension. J Am Coll Cardiol. 2004;43:764–70. doi: 10.1016/j.jacc.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 29.Sutton TM, Stewart RA, Gerber IL, et al. Plasma natriuretic peptide levels increase with symptoms and severity of mitral regurgitation. J Am Coll Cardiol. 2003;41:2280–7. doi: 10.1016/s0735-1097(03)00486-8. [DOI] [PubMed] [Google Scholar]
- 30.Vanderheyden M, Goethals M, Verstreken S, et al. Wall stress modulates brain natriuretic peptide production in pressure overload cardiomyopathy. J Am Coll Cardiol. 2004;44:2349–54. doi: 10.1016/j.jacc.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 31.Troughton RW, Prior DL, Pereira JJ, et al. Plasma B-type natriuretic peptide levels in systolic heart failure: Importance of left ventricular diastolic function and right ventricular systolic function. J Am Coll Cardiol. 2004;43:416–22. doi: 10.1016/j.jacc.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 32.Rudiger A, Gasser S, Fischler M, Hornemann T, von Eckardstein A, Maggiorini M. Comparable increase of B-type natriuretic peptide and amino-terminal pro-B-type natriuretic peptide levels in patients with severe sepsis, septic shock, and acute heart failure. Crit Care Med. 2006;34:2140–4. doi: 10.1097/01.CCM.0000229144.97624.90. [DOI] [PubMed] [Google Scholar]
- 33.ten Wolde M, Tulevski II, Mulder JW, et al. Brain natriuretic peptide as a predictor of adverse outcome in patients with pulmonary embolism. Circulation. 2003;107:2082–4. doi: 10.1161/01.CIR.0000070020.79932.DB. [DOI] [PubMed] [Google Scholar]
- 34.Kucher N, Printzen G, Goldhaber SZ. Prognostic role of brain natriuretic peptide in acute pulmonary embolism. Circulation. 2003;107:2545–7. doi: 10.1161/01.CIR.0000074039.45523.BE. [DOI] [PubMed] [Google Scholar]
- 35.Pruszczyk P, Kostrubiec M, Bochowicz A, et al. N-terminal pro-brain natriuretic peptide in patients with acute pulmonary embolism. Eur Respir J. 2003;22:649–53. doi: 10.1183/09031936.03.00023303. [DOI] [PubMed] [Google Scholar]
- 36.Knudsen CW, Omland T, Clopton P, et al. Impact of atrial fibrillation on the diagnostic performance of B-type natriuretic peptide concentration in dyspneic patients: An analysis from the Breathing Not Properly multinational study. J Am Coll Cardiol. 2005;46:838–44. doi: 10.1016/j.jacc.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 37.Rossi A, Enriquez-Sarano M, Burnett JC, Jr, Lerman A, Abel MD, Seward JB. Natriuretic peptide levels in atrial fibrillation: A prospective hormonal and Doppler-echocardiographic study. J Am Coll Cardiol. 2000;35:1256–62. doi: 10.1016/s0735-1097(00)00515-5. [DOI] [PubMed] [Google Scholar]
- 38.Horwich TB, Hamilton MA, Fonarow GC. B-type natriuretic peptide levels in obese patients with advanced heart failure. J Am Coll Cardiol. 2006;47:85–90. doi: 10.1016/j.jacc.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 39.Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: Results from the Dallas Heart Study. Circulation. 2005;112:2163–8. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 40.Mehra MR, Uber PA, Park MH, et al. Obesity and suppressed B-type natriuretic peptide levels in heart failure. J Am Coll Cardiol. 2004;43:1590–5. doi: 10.1016/j.jacc.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 41.Wang TJ, Larson MG, Levy D, et al. Impact of obesity on plasma natriuretic peptide levels. Circulation. 2004;109:594–600. doi: 10.1161/01.CIR.0000112582.16683.EA. [DOI] [PubMed] [Google Scholar]
- 42.Rivera M, Cortés R, Salvador A, et al. Obese subjects with heart failure have lower N-terminal pro-brain natriuretic peptide plasma levels irrespective of aetiology. Eur J Heart Fail. 2005;7:1168–70. doi: 10.1016/j.ejheart.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Daniels LB, Clopton P, Bhalla V, et al. How obesity affects the cut-points for B-type natriuretic peptide in the diagnosis of acute heart failure. Results from the Breathing Not Properly multinational study. Am Heart J. 2006;151:999–1005. doi: 10.1016/j.ahj.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: Impact of age and gender. J Am Coll Cardiol. 2002;40:976–82. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 45.Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: Determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345–53. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dokainish H, Zoghbi WA, Lakkis NM, Quinones MA, Nagueh SF. Comparative accuracy of B-type natriuretic peptide and tissue Doppler echocardiography in the diagnosis of congestive heart failure. Am J Cardiol. 2004;93:1130–5. doi: 10.1016/j.amjcard.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 47.Arques S, Roux E, Sbragia P, et al. Accuracy of tissue Doppler echocardiography in the emergency diagnosis of decompensated heart failure with preserved left ventricular systolic function: Comparison with B-type natriuretic peptide measurement. Echocardiography. 2005;22:657–64. doi: 10.1111/j.1540-8175.2005.40076.x. [DOI] [PubMed] [Google Scholar]
- 48.Mak GS, DeMaria A, Clopton P, Maisel AS. Utility of B-natriuretic peptide in the evaluation of left ventricular diastolic function: Comparison with tissue Doppler imaging recordings. Am Heart J. 2004;148:895–902. doi: 10.1016/j.ahj.2004.02.016. [DOI] [PubMed] [Google Scholar]