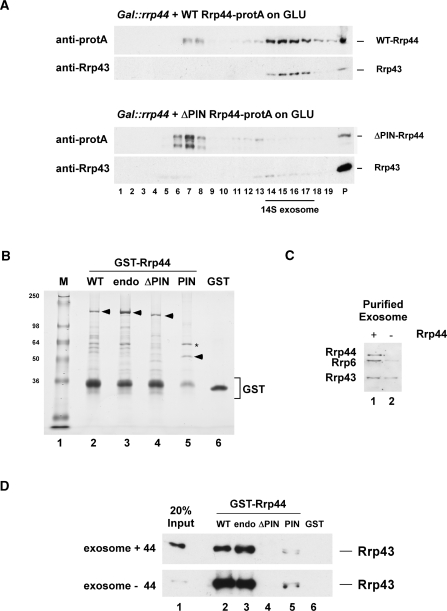
Figure 7.
The N-terminal PIN domain in Rrp44 mediates protein–protein interactions. (A) Sedimentation behavior of WT and Rrp44-ΔPIN on glycerol gradients. Gal::rrp44 strains transformed with plasmids encoding protA-tagged Rrp44 [full-length (WT) or ΔPIN] were grown on glucose for 8.5 h to deplete endogenous Rrp44. Protein extracts from 27.5 ODs of cells were then separated on linear 10–30% glycerol gradients and analyzed by western blotting. (B) Recombinant, GST-tagged WT Rrp44 (WT), Rrp44-endo (endo), Rrp44-ΔPIN (ΔPIN), the PIN domain alone (PIN) and free GST were expressed in E. coli and purified on a glutathione sepharose column. The proteins (indicated by arrowheads) were separated on an 8% polyacrylamide/SDS gel and stained with Coomassie blue. Asterisk: E. coli GroEL. (C) Western blot analysis of TAP-purified exosomes from Csl4-TAP strains grown at 25°C. Exosomes were purified on IgG sepharose at 150 mM NaCl (lane 1) or treated with 800 mM MgCl2 to dissociate endogenous Rrp44 (lane 2). TEV eluates (2.5 μl) were separated on an 8% polyacrylamide/SDS gel and analyzed by immunoblotting with anti-Rrp44, anti-Rrp6 and anti-Rrp43 antibodies. (D) GST-pull down experiment. The GST-tagged Rrp44 constructs or free GST (B) were coupled to glutathione sepharose beads and incubated with purified yeast exosomes (C). Bound material was eluted under denaturing conditions, separated on an 8% polyacrylamide/SDS gel and analyzed by immunoblotting with an anti-Rrp43 antibody.