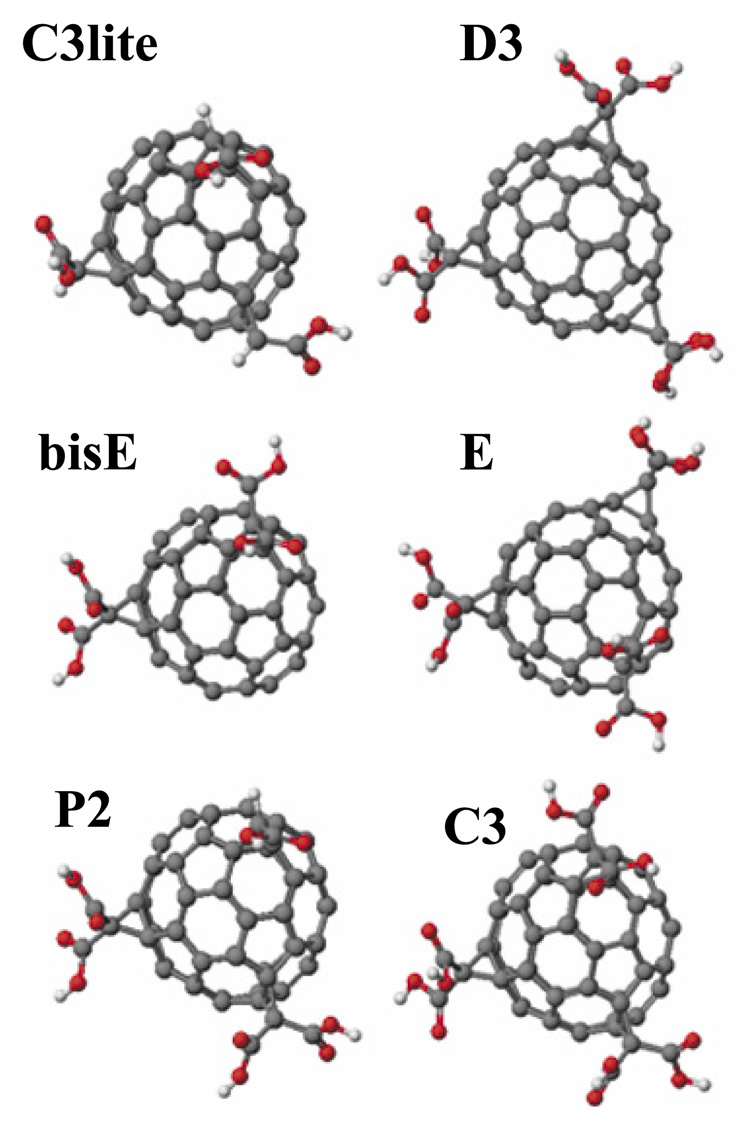
Figure 1.
Structural models of the carboxyfullerenes employed in the present study. Molecular models, geometry optimization, and energy minimization for all molecules were carried out to arrive at the indicated structural models. The selection of these compounds was based on structural variability that illustrates the functional dependence on two molecular structural aspects: First, the number of carboxylic acid groups attached to the fullerene moiety was increased from 3 to 6 in the order C3lite < bisE < P2 < C3; and secondly, the distribution of a fixed number of carboxylic groups over the fullerene surface was varied in the hexa isomers (C3, E, and D3).
The IUPAC nomenclatures of these compounds are: bisE, 3’H,3’’H-dicyclopropa[1,9:21,40]-(C60 Ih)[5,6]fullerene-3’,3’,3’’,3’’-tetracarboxylic acid; C3lite, 3’H,3’’H,3’’’H tricyclopropa[1,9:21,40:44,45]-(C60-Ih)[5,6]fullerene-3’,3’’,3’’’-tricarboxylic acid; P2, 3’H,3’’H,3’’’H-tricyclopropa[1,9:21,40:44,45]-( C60-Ih)[5,6]fullerene-3’,3’,3’’,3’’,3’’’- pentacarboxylic acid; E, 3’H,3’’H,3’’’H-tricyclopropa[1,9:21,40:34,35]-( C60-Ih)[5,6]fullerene-3’,3’,3’’,3’’,3’’’,3’’’-hexacarboxylic acid; C3, 3’H,3’’H,3’’’H-tricyclopropa[1,9:21,40:44,45]-(C60-Ih)[5,6]fullerene-3’,3’,3’’,3’’,3’’’,3’’’-hexacarboxylic acid; D3, 3’H,3’’H,3’’’H-tricyclopropa[1,9:34,35:43,57]-(C60-Ih)[5,6]fullerene-3’,3’,3’’,3’’,3’’’,3’’’-hexacarboxylic acid.