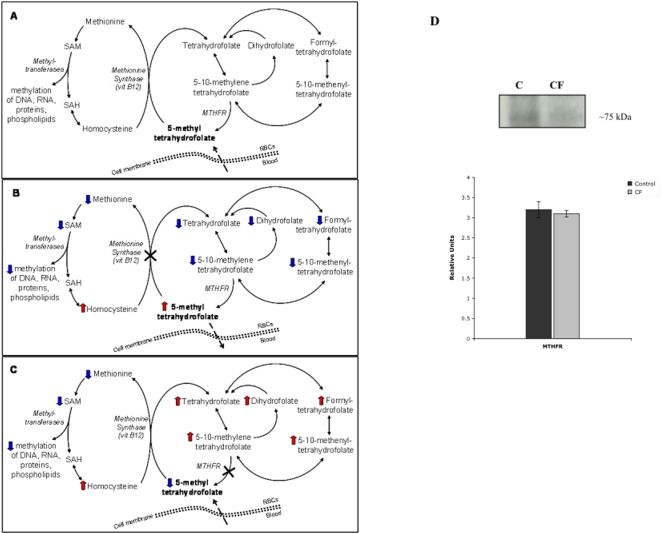
Figure 2. The intracellular folate metabolism.
(A) Human cells receive exogenous 5-methyltetrahydrofolate (5-MTHF) from the bloodstream and produce endogenous 5-MTHF through the irreversible methylenetetrahydrofolate reductase (MTHFR) reaction that catalyzes the reduction of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate. 5-MTHF serves as methyl donor in the remethylation of homocysteine to methionine, which in turn is converted to S-adenosylmethionine (SAM). The transmethylation reaction, which requires vitamin B12 and the methionine synthase enzyme, converted 5-MTHF in tetrahydrofolate (THF). As 5-MTHF is a poor substrate for folylpolyglutamate synthetase, this enzyme adds glutamyl residues to non-methylated folates, guaranteeing the intracellular folate retention. (B) A reduced activity of the methionine synthase activity or a deficit of vitamin B12 causes a reduction of the intracellular folates. In fact, 5-MTHF leaks out of cells because cells cannot accumulate it, being monoglutamised and therefore shorter than other intracellular folates. Conversely, (C) a reduced activity of the methylenetetrahydrofolate reductase (MTHFR) causes the non-methylated folate accumulation, because these folates are polyglutamised by folylpolyglutamate synthase. (D) Immunoblot analysis of methythertrahydrofolate reductase (MTHFR) protein expression in red cells from healthy controls and in CF patients. One representative gel of other 20 with similar results. Graph reporting the immuno-blot analysis for the quantification of MTHFR expression as detected by densitometry in normal controls (black bar) and in CF patients (gray bar); data are presented as percentage of baseline values (n = 20).