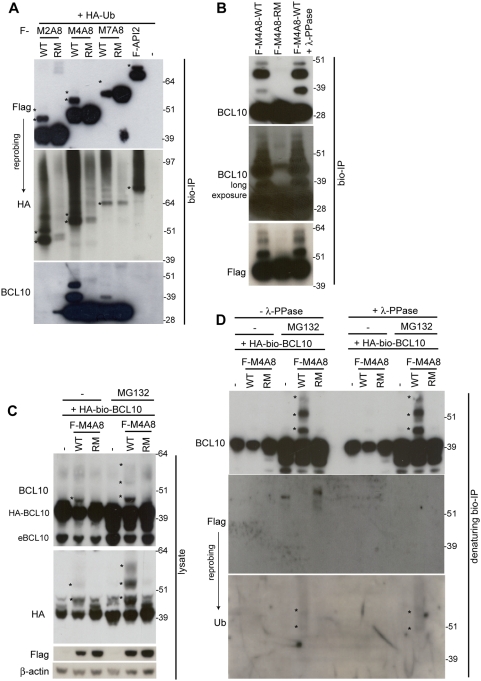
Figure 3. BCL10 is a target of the RING E3 ubiquitin ligase activity of MALT1-API2 variants with MALT1 Ig-like domains.
A–B, Flag-bio-tagged M2A8, M4A8 and M7A8 (wild-type (WT) or RING mutant (RM)), API2 and empty vector were transiently expressed together with HA-Ub in HEK-293T. Protein complexes were precipitated with streptavidin-coated paramagnetic beads and analyzed with α-Flag, α-HA (after stripping of α-Flag) and α-BCL10. A, Mono- and di-ubiquitinated bands of WT MALT1-API2 and API2 are indicated with asterisks in the α-Flag and α-HA panel. B, Where indicated, the precipitated protein complex was treated with λ-PPase to remove phosphorylation. C–D, BCL10 ubiquitination by M4A8 becomes more pronounced after inhibition of the proteasome. HA-bio-tagged BCL10 was transiently overexpressed in HEK-293T together with Flag-tagged M4A8 (WT or RM) or empty vector. Where indicated, cells were pretreated with MG132 to inhibit to proteasome. C, Cell lysates were immunodetected with α-BCL10 and α-HA (both for HA-BCL10), α-Flag and α-β-actin. BCL10 ubiquitination is indicated with asterisks. D, Following denaturation of the cell lysates, HA-bio-tagged BCL10 was precipitated via streptavidin pull-down and where indicated, treated with λ-PPase to remove phosphorylation. The bio-IPs were then immunodetected with α-BCL10, α-Flag (to check if the denaturation was complete) and α-Ub. BCL10 ubiquitination is indicated with asterisks. Molecular weight standards are in kDa.