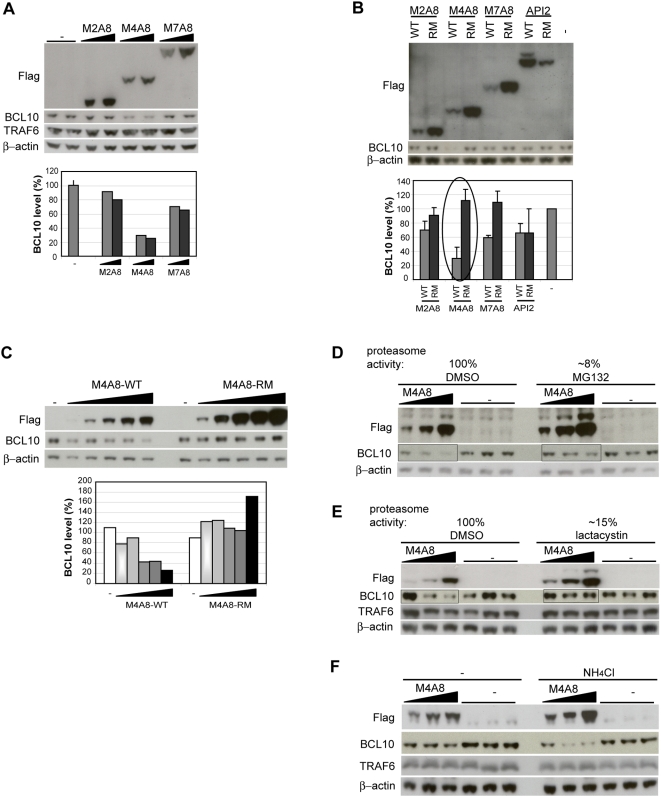
Figure 4. M4A8 induces BCL10 degradation by the proteasome.
A, M4A8, and to a lesser extent M7A8, induces BCL10 degradation. Cell lysates of HEK-293T cells, transiently transfected with empty vector or increasing concentrations of Flag-tagged M2A8, M4A8 and M7A8 were immunodetected with α-Flag, α-BCL10, α-TRAF6 and α-β-actin. The BCL10 protein level (%) is compared to empty vector-transfected cells after normalization to β-actin. B, M4A8 requires a functional RING domain to induce BCL10 degradation. HEK-293T cells, transiently transfected with empty vector or Flag-tagged M2A8, M4A8, M7A8 and API2 (WT or RM) were lysed and immunodetected with α-Flag, α-BCL10 and α-β-actin. The BCL10 protein level (%) is compared to empty vector-transfected cells after normalization to β-actin and represents the mean of three independent experiments, with indicated standard deviation. C, HEK-293T cells were transiently transfected with empty vector or increasing concentrations of Flag-tagged WT or RM M4A8. Cell lysates were analyzed with α-Flag, α-BCL10 and α-β-actin. The BCL10 protein level (%) is compared to empty vector-transfected cells after normalization to β-actin. D–E, Proteasome inhibition reduces the degradation of BCL10 and of M4A8 itself. HEK-293T cells, transiently transfected with empty vector or increasing concentrations of Flag-tagged M4A8 were pretreated with DMSO, MG132 (D) or lactacystin (E) prior to harvesting. Cell lysates were analyzed with α-Flag, α-BCL10 and α-β-actin. F, Degradation of BCL10 or MALT1-API2 does not require the lysosome. HEK-293T cells, transiently transfected with empty vector or increasing concentrations of Flag-tagged M4A8 were left untreated or pretreated with NH4Cl prior to harvesting. Cell lysates were analyzed with α-Flag, α-BCL10, α-TRAF6 and α-β-actin.