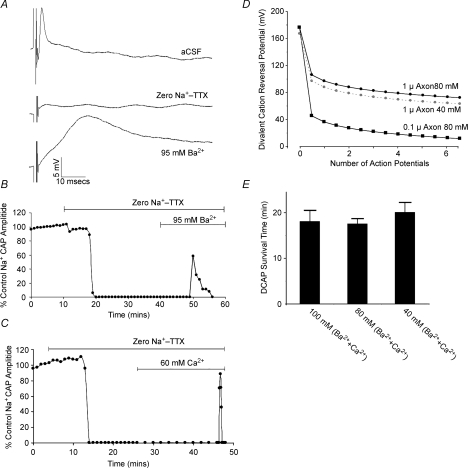
Figure 2. Features of the DCAP in P8–P12 rat optic nerve.
A, high concentrations of a single divalent cation can produce a DCAP. Top, a normal compound action potential recorded in aCSF. Middle, block of this action potential in zero-Na+–TTX solution. Bottom, a large-amplitude, slow-conducting DCAP is recorded in the presence of 95 mm Ba2+. B, action potential amplitude is plotted against time, demonstrating the decline in the DCAP over an ∼10 min time course. C, a similar protocol showing the time-course of a DCAP recorded in 60 mm Ca2+. Switching to zero-Na+–TTX solution produced block of the normal action potential and changing to 60 mm extracellular Ca2+ resulted in a short-lasting DCAP. D, the divalent cation reversal potential in axons calculated assuming a starting axoplasmic concentration of 100 nm, an extracellular divalent cation concentration of 90 mm, a resting membrane potential of –70 mV, a DCAP amplitude of 100 mV and a membrane capacitance of 1 μF cm−2. It is also assumed that there is no significant extrusion of divalent cations entering during the DCAP and that there is no influx into the axon when the axolemma is at rest. Given these assumptions, it is apparent that a small number of divalent cation action potentials have a dramatic affect upon the divalent cation reversal potential, in particular for the smaller diameter axons (0.1 μm, filled squares). Reducing the extracellular divalent cation concentration to 40 mm has minimal effect upon the time course of the collapse of the reversal potential (grey circles, plotted for a 1 μm axon). E, the time between the first appearance of a DCAP and its failure (survival time) in various high divalent cation conditions, showing that varying the total divalent cation concentration between 40 and 100 mm had no significant affect, as predicted in D.