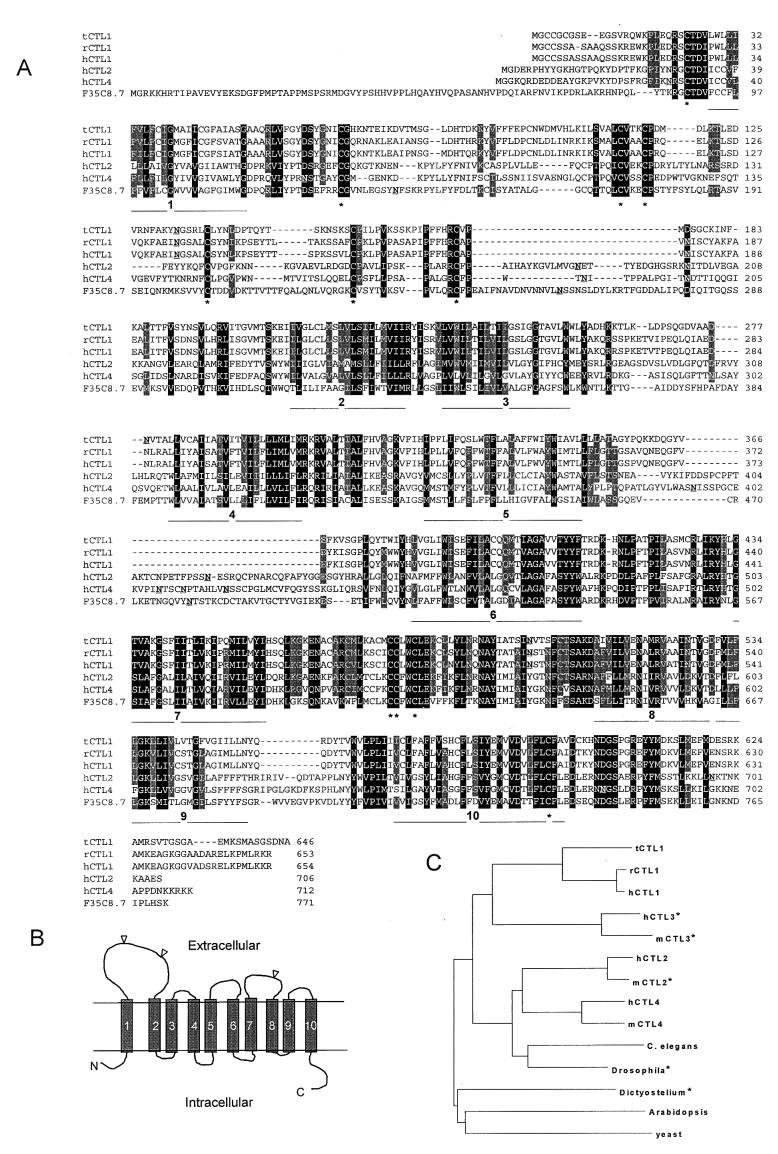
Figure 2.
Sequence alignments of CTL proteins and a model of their membrane topology. (A) Amino acid sequence alignment of CTL1 from Torpedo (tCTL1), rat (rCTL1), and human (hCTL1) with two homologous proteins, hCTL2 and hCTL4, and the single homologous C. elegans protein, F35C8.7. Black shading indicates 100% conservation and grey shading indicates 80% conservation (blosum 62). Stretches of hydrophobic amino acids that may form TMDs are indicated under the alignments and numbered. Potential N-glycosylation sites are underlined in each sequence. Conserved cysteines are marked with asterisks under the alignment. (B) Structural model of CTL1. (C) Dendrogram of tCTL1 and related proteins obtained by using clustalw. No prokaryote homologs have been found. The asterisks indicate that only partial protein sequences were used for the analysis.