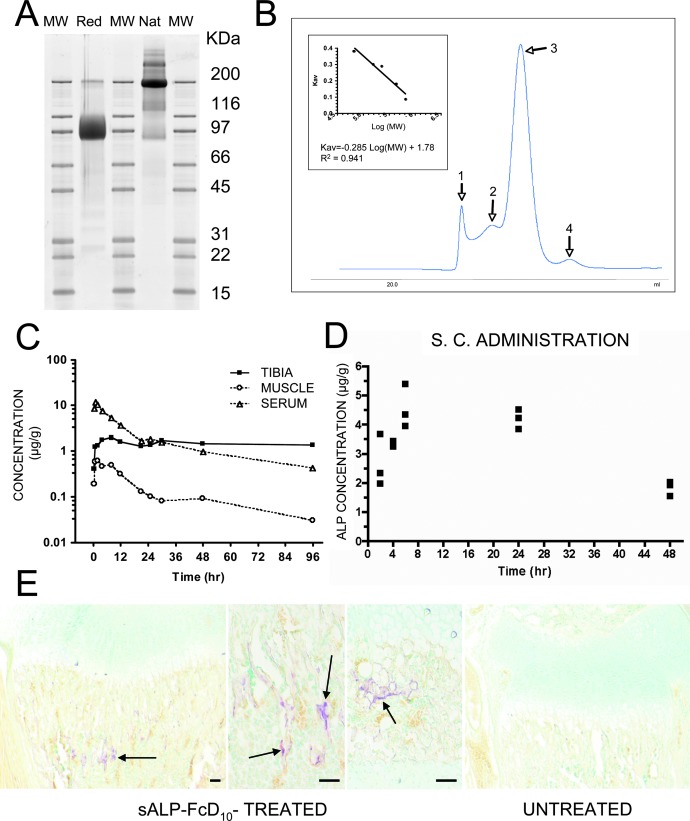
FIG. 1.
Purification and properties of recombinant sALP-FcD10 and pharmacokinetic and tissue distribution studies. (A) SDS-PAGE of purified sALP-FcD10. Protein purified by Protein A-Sepharose affinity chromatography was analyzed by SDS-PAGE and bands were stained with Sypro Ruby. sALP-FcD10 migrated as the major species with an apparent molecular mass of ∼90,000 Da under reducing conditions (Red) and ∼200,000 Da under nonreducing, native conditions (Nat). (B) Characterization of sALP-FcD10 by molecular sieve chromatography under nondenaturing conditions. Purified sALP-FcD10 protein (2 mg) was resolved on a calibrated column of Sephacryl S-300. The principal form of sALP-FcD10 (Peak 3), consisting of 80% of the total material deposited on the column, eluted with a molecular mass of 370,000 Da consistent with a tetrameric structure. When analyzed by SDS-PAGE in the presence of dithiothreitol (DTT), the material in peak 3 migrated with an apparent molecular mass of a monomer. In the absence of DTT, the protein migrated with the mobility of a dimer. (C) Concentrations of radiolabeled sALP-FcD10 in serum, tibia, and muscle, expressed as micrograms per gram tissue (wet weight), after a single intravenous bolus of 5 mg/kg in adult WT mice (n = 3). (D) Serum concentrations of radiolabeled sALP-FcD10 as a function of time after a single subcutaneous injection of 3.7 mg/kg in 1-day-old WT mice (n = 3). (E) Histochemical staining for ALP activity in the long bones of sALP-FcD10–treated Akp2−/− mice. Proximal tibia with EzRT compared with an age-matched untreated Akp2−/− mouse. Arrows show areas of ALP activity staining. Bars: 100 μm.