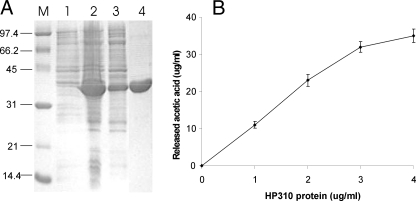
FIGURE 5.
Demonstration of deacetylase activity of HP310 protein. A, SDS-PAGE of the purified protein HP310-His6. His-tagged HP310 was overexpressed in E. coli in the presence of 1 mm isopropyl 1-thio-β-d-galactopyranoside. Lanes 1 and 2, proteins extracted from whole cells without and with isopropyl 1-thio-β-d-galactopyranoside induction. Lane 3, the purification intermediate (supernatant from a 45,000 × g centrifugation). Lane 4, the purified HP310-His6 protein. Lane M, protein standards. B, deacetylase activity of HP310 protein using H. pylori PG as a substrate. 1 mg of the isolated PG was incubated with various amounts of the purified HP310 protein (as indicated in x axis) for 4 h at 37 °C. The PG deacetylase activity is demonstrated as release of acetic acid during the reaction. The data are the means of three experiments with standard deviations.