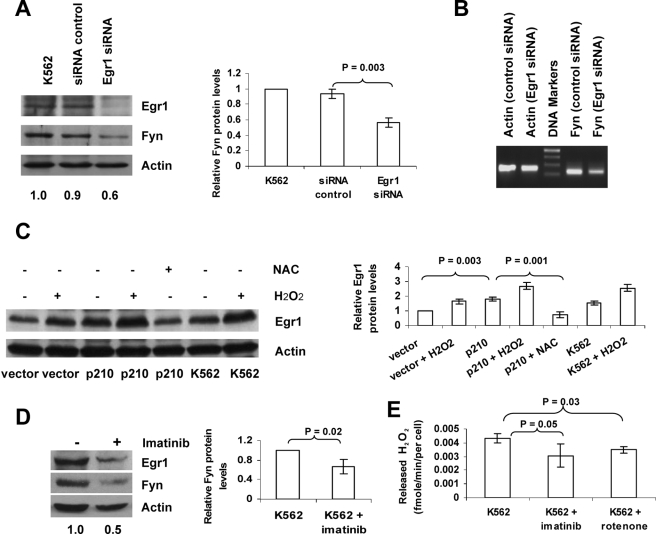
FIGURE 5.
Increased Fyn expression in Bcr-Abl1-expressing cells results from ROS-dependent activation of Egr1. A and B, siRNA-mediated inhibition of Egr1 leads to decreased Fyn expression. K562 cells were transfected once with Egr1 siRNA or control, followed by a second transfection after 24 h. Protein levels of Egr1 and Fyn were assessed by Western blotting, Densitometry was conducted (Fyn:actin ratio normalized to control) on three independent experiments and is depicted in the bar graph. Fyn mRNA levels were measured by PCR. C, hydrogen peroxide induces Egr1, whereas NAC blocks up-regulation of Egr1 in BaF3 and K562 cells. After treatment with 50 μm H2O2 or with 24 mm NAC for 24 h, Egr1 protein levels were assessed by Western blot. Densitometric values from three independent experiments are depicted in the bar graph to the right of the Western blot. D, inhibition of BCR-ABL1 decreases Egr1 and Fyn protein expression in K562 cells. Cells were treated with diluent or 0.25 μm imatinib mesylate for 24 h. Levels of Egr1 and Fyn protein were measured by Western blotting. All blots above were reprobed with an actin antibody to ensure equal loading. The numerical values listed below the bands represent a ratio of Fyn protein expression to actin protein expression. E, inhibition of BCR-ABL1 or electron transfer decreases hydrogen peroxide released from K562 cells. Cells were treated with 0.25 μm imatinib mesylate for 24 h or treated with 0.10 μm rotenone for 1 h, released hydrogen peroxide levels measured by the Amplex Red and horseradish peroxidase method described under “Experimental Procedures.”