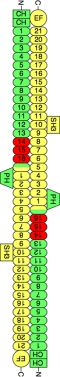
FIGURE 1.
B14–16 region of the spectrin tetramer. The diagram shows the arrangement of α2- and β2-spectrins in the spectrin tetramer along with the domain organization of each subunit. α-Spectrin is colored yellow, and β-spectrin is colored green. Spectrin repeats are indicated by number. Shown in red are the spectrin repeats of β-spectrin that were crystallized. The Src homology 3 (SH3) domain in α2-spectrin is an insert in spectrin repeat 10. EF refers to the EF-hand domains at the C terminus of α2-spectrin, which bind to calcium. In β-spectrin, the CH motifs bind to actin, whereas the pleckstrin homology (PH) domain binds to lipids. The end-to-end association of α- and β-spectrin is mediated by partial spectrin repeats that form a three-helix bundle consisting of one helix from α-spectrin and two helices from β-spectrin.