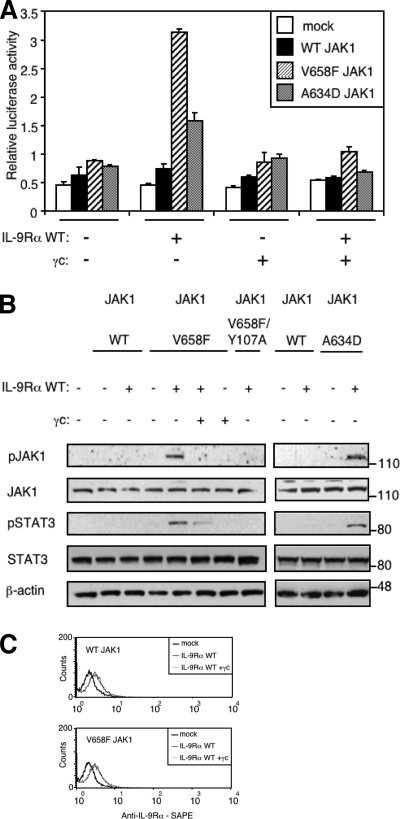
FIGURE 3.
Co-expression of γc inhibits IL-9Rα-mediated JAK1 phosphorylation and STAT3 activation. A, HEK293 cells were transiently co-transfected with empty vector, JAK1 wild-type or V658F, γc, or/and IL-9Rα in addition to the STAT3-responsive luciferase reporter pGL3-pap1. 24 h post-transfection cells were subjected to a luciferase assay. Results are the mean ± variation of duplicate samples. Similar results were obtained in three independent experiments. B, HEK293 cells were transiently co-transfected with different empty vector, JAK1 constructs, γc, or/and IL-9Rα. 24 h post-transfection, 106 cells were lysed and subjected to Western blot analysis. Phosphorylation of JAK1 and STAT3 was detected using specific anti-pJAK1 Tyr-1022/1023 and anti-pSTAT3 Tyr-705 antibodies. Membranes were reprobed with anti-JAK1, anti-STAT3 and anti-β-actin antibodies as control. Similar results were obtained in two independent experiments. C, HEK293 cells were transiently co-transfected with empty vector, JAK1 wild-type or V658F, and IL-9Rα with or without γc in addition to the STAT3-responsive luciferase reporter pGL3-pap1. One day post-transfection, an aliquot of cells was used to assess cell surface expression of IL-9Rα by FACS analysis using anti-human IL-9Rα antibody followed with phycoerythrin-conjugated streptavidin (SAPE).