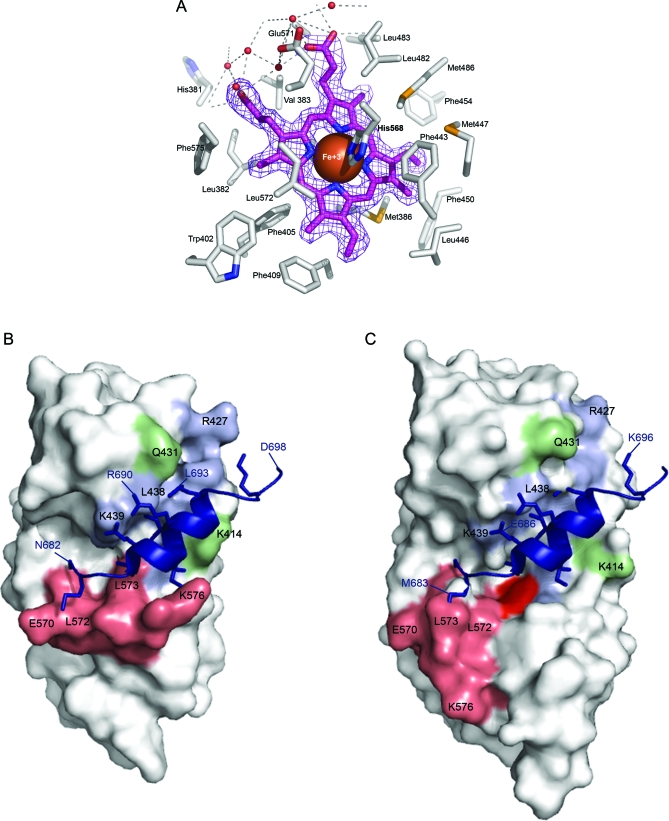
Figure 8. Effects of Heme Binding on the REV-ERBβ Ligand-Binding Pocket and Co-Repressor Binding Groove.
(A) A detailed view of the heme-bound REV-ERBβ ligand-binding pocket. Electron density of the porphyrin ring and iron is closely bounded by hydrophobic sidechains of the ligand-binding pocket.
The hydrophilic propionate groups of heme interact with water molecules (red spheres) near the surface of the protein. Model of Apo-(B) [21] and heme-bound (C) REV-ERBβ LBD structures in complex with a SMRT ID-I co-repressor peptide [101]. Peptide residues (N682–D698) are in dark blue, groove residues previously identified as being important in peptide binding are in blue (F409, V417, K421, R427, V435, L438, K439) [21,59,60], residues in H11 are in salmon (which also include residues previously identified as important to co-repressor binding; L572, F575, K576), heme is in red, and other residues in the groove that shift upon heme binding are in green (K414, Q431).