Abstract
Background
Several in vitro studies have looked at the effect of medicinal plant extracts against Helicobacter pylori (H. pylori). Regardless of the popular use of Byrsonima crassa (B. crassa) as antiemetic, diuretic, febrifuge, to treat diarrhea, gastritis and ulcers, there is no data on its effects against H. pylori. In this study, we evaluated the anti-H. pylori of B. crassa leaves extracts and its effects on reactive oxygen/nitrogen intermediates induction by murine peritoneal macrophages.
Methods
The minimal inhibitory concentration (MIC) was determined by broth microdilution method and the production of hydrogen peroxide (H2O2) and nitric oxide (NO) by the horseradish peroxidase-dependent oxidation of phenol red and Griess reaction, respectively.
Results
The methanolic (MeOH) and chloroformic (CHCl3) extracts inhibit, in vitro, the growth of H. pylori with MIC value of 1024 μg/ml. The MeOH extract induced the production H2O2 and NO, but CHCl3 extract only NO.
Conclusion
Based in our results, B. crassa can be considered a source of compounds with anti-H. pylori activity, but its use should be done with caution in treatment of the gastritis and peptic ulcers, since the reactive oxygen/nitrogen intermediates are involved in the pathogenesis of gastric mucosal injury induced by ulcerogenic agents and H. pylori infections.
Background
Helicobacter pylori (H. pylori) is a spiral-shaped bacterium that colonizes the stomach of the half of all people worldwide [1]. Once a person is infected, the organism can live in the stomach indefinitely and may not cause clinical illness. It is still not clear how H. pylori are transmitted or why some people infected with its bacteria become sick and others do not [2]. Studies have also shown an association between long-term infection with H. pylori and the development of gastric adenocarcinoma [3,4].
Therapy for H. pylori infection consists of 1–2 weeks of one or two effective antibiotics, such as amoxicillin, tetracycline, metronidazole, or clarithromycin, plus either ranitidine bismuth citrate, bismuth subsalicylate, or a proton pump inhibitor [5]. Many clinical treatment trials involving patients with H. pylori infection and gastric or duodenal ulcers show that curing the infection is associated with a marked reduction in ulcer recurrence rates [6]. However, eradication by the triple therapy is not always successful and the acquisition by H. pylori resistant to antibiotics could represent a serious problem that may reduce treatment efficacy [7]. Considering that eradication therapies can be ineffective and undesirable side effects may occur, the search for new drugs for the development of alternative therapies is very important [1]. Plant extracts are among the attractive sources of new drugs and have been shown to produce promising results in the treatment of gastric ulcers [8-11].
The genus Byrsonima, which is composed of approximately 150 species, belongs to the Malpighiaceae family and is widely distributed throughout tropical America [12,13]. This family is constituted by approximately 800 species distributed in 60 genera and about 50% of these species are concentrated in Brazil [14]. In traditional Brazilian medicine, Byrsonima crassa (B. crassa) is used as antiemetic, diuretic, febrifuge, to treat diarrhea, gastritis and ulcer [15].
The potential antiulcerogenic of B. crassa leaves extracts were demonstrated by Sannomiya et al. [16]. The results of this research showed that methanolic (MeOH) extract provided better gastroprotective activity than chloroformic (CHCl3) extract. The presence of amentoflavone, quercetin derivatives and catechins in the MeOH extract were suggested to contribute for the gastroprotective activity since these compounds were reported to inhibit lipid peroxidation as well as possess a very potent antioxidant activity [16,17].
Several in vitro studies have looked at the effect of medicinal plant extracts against H. pylori [18-21]. This bacteria induces inflammation, infiltration and activation of immune cells, accumulation of reactive oxygen species, and oxidative DNA damage in the gastric mucosa [22-24]. The antimicrobial compounds from plants may inhibit bacterial growth by different mechanisms than those presently used antimicrobials, and could therefore be of clinical value in the treatment of resistant microbial strains, including H. pylori [25].
Despite of the popular use of B. crassa as a medicinal plant, there is no data on its antimicrobial activity and immunostimulatory effects. In this study, we evaluated, in vitro, the anti-H. pylori of B. crassa leaves extracts (MeOH and CHCl3) and its immunostimulatory effects in murine immune system by determination of oxygen (H2O2) and nitrogen (NO) intermediates reactive.
Methods
Plant material
B. crassa Nied. (Malpighiaceae) leaves were collected at Porto Nacional, Tocantins State, Brazil and authenticated by Prof. Eduardo Ribeiro dos Santos. A voucher specimen (Nr. 3377) was deposited at the Herbarium of the Tocantins University.
Extraction and preparation of extract solutions
The aerial parts (2.0 kg of leaves) obtained were dried (at 40°C for 4 days) and powdered. The dry powdered material was macerated three times with 2 liters of chloroform and methanol successively at room temperature and left for 48 h in the respective solvent. The solvents were filtered and evaporated at 60°C under reduced pressure providing CHCl3 (53.8 g) and MeOH (158.3 g) extracts. The yields (w/w) for the CHCl3 and MeOH extracts from the air-dried and powdered leaves of B. crassa leaves were 2.7 and 7.9%, respectively [16]. Stock solutions of plant extracts (50 mg/ml) were prepared in dimethyl sulfoxide (DMSO) and stored at -20°C. Dilutions of the stock solutions were made in Brain Heart Infusion (BHI) plus 10% fetal bovine serum (FBS) for antimicrobial activity and in potassium phosphate buffer or RPMI-1640 medium for measurement of H2O2 and NO production by peritoneal macrophages. Fresh solutions were prepared for each experiment.
Bacterial strain
H. pylori ATCC 43504, metronidazole resistant (MtzR) and amoxicillin susceptible (AmxS), was obtained from the American Type Culture Collection (Manassas, VA, USA). The bacterium was cultured in Columbia agar containing 5% sheep blood at 36–37°C for 3 days, in 5% O2, 10% CO2, 85% N2 atmosphere.
Antimicrobial activity
The wells of a 96-well microplate were filled with 100 μl of various concentrations of the extracts. Same volume of H. pylori suspension (about 106 cfu/ml) was added to each well. The absorbance was determined using an automatic ELISA microplate reader (Spectra & Rainbow Readers, Tecan) adjusted at 620 nm. The microplate was incubated at 36–37°C for 3 days, under microaerophilic atmosphere, agitated and the absorbance was read again in the reader at the same wavelength. The absorbencies were compared to the values obtained before incubation to detect an increase in bacterial growth. The lowest concentration of the test extract resulting in inhibition of bacterial growth, at least, more than 90%, was taken as the minimal inhibitory concentration (MIC). Amoxicillin and metronidazole were used as reference antimicrobial.
Animals
Experiments involving Swiss mice (6–8 weeks old, 18 to 25 g) were performed in accordance with the regulations of Research Ethics Committee (01/2005), Faculty of Pharmaceutical Sciences, Unesp, São Paulo, Brazil.
Peritoneal macrophages
Resident and thioglycollate-elicited peritoneal exudate cells were obtained from mice following intraperitoneal injection of 3 ml thioglycollate medium (3.0 g/100 ml) and lavage of the peritoneal cavity with 5 ml of 10 mM phosphate-buffered saline (PBS), pH 7.2, 3–4 days later. The proportion of macrophages in the peritoneal exudate was determined by cell staining with May-Gruenwald-Giemsa. Cell preparations contained more than 95% macrophages. The cells were washed twice with PBS and resuspended in appropriate medium for each test.
Macrophages viability
For the determination of the concentrations of extracts that do not cause cell death, the cytotoxic assay was performed as described. Macrophages (106 cells/ml) were suspended in RPMI-1640 containing 5% heat-inactivated FBS, 100 IU/ml penicillin, 100 μg/ml streptomycin and 50 mM 2-mercaptoethanol. The suspension (100 μl) was added to each well of a 96-well microplate and the cells were incubated at 37°C in a humidified atmosphere containing 5% CO2. After 1 h, the wells were washed and adhering cells exposed to different concentrations of methanolic or chloroformic extract for 1 and 24 h. The test was accompanied by a viability positive control (RPMI plus cells) and negative control (RPMI plus extract). Finally, neutral red (NR) assay [26] was performed and the absorbance at 540 nm (reference filter 620 nm) determined using an automatic microplate reader.
Measurement of H2O2
Production H2O2 was measured by the horseradish peroxidase (HRP)-dependent oxidation of phenol red [27]. Macrophages (2 × 106 cells/ml) were suspended in 10 mM potassium phosphate buffer containing 140 mM NaCl, 5.5 mM dextrose, 0.56 mM phenol red and 0.01 mg/ml HRP, pH 7.4. Briefly, 100 μl of this suspension was added to each of a 96-well culture tissue plate and exposed to methanolic and chloroformic extracts (concentrations of extracts that do not cause cell death), for 1 h (time for the H2O2 assay) at 37°C in a 5% CO2 atmosphere. The reaction was stopped by the addition of 10 μl of 1 N NaOH and the absorbances were read at 620 nm using a microplate reader. The results are reported as nmol/2 × 105 cells. The experiment was accompanied by a positive control (buffer plus macrophages and 200 nM phorbol myristate acetate, PMA) and negative control (buffer plus macrophages).
Measurement of NO
NO synthesis was determined by measuring the accumulation of nitrite, a stable metabolite of NO, using the Griess reaction [28]. Macrophages (100 μl) in at 5 × 106 cells/ml in RPMI containing 5% heat-inactivated FBS, 100 IU/ml penicillin, 100 μg/ml streptomycin and 50 mM 2-mercaptoethanol, were added to each well of a 96-well cell culture plate and exposed to methanolic and chloroformic extracts (concentrations that do not cause cell death), for 24 h (time for the NO assay) at 37°C in a 5% CO2 atmosphere. After incubation, 50 μl aliquots of culture supernatant were mixed with an equal volume of Griess reagent and incubated at room temperature for 10 min. Absorbance at 540 nm was measured using a microplate reader. The results are reported as μmol/5 × 105 cells. Each experiment was accompanied by a positive control (RPMI plus macrophages and 10 μg/ml lipopolysaccharide, LPS) and a negative control (RPMI and macrophages).
Statistical Analysis
The results are expressed as means ± SD (Standard Deviation). All tests were perform in triplicate and repeated at least three times. Statistical difference between groups was determined by one-way analysis of variance (ANOVA). A p-value < 0.05 was considered statistically significant.
Results
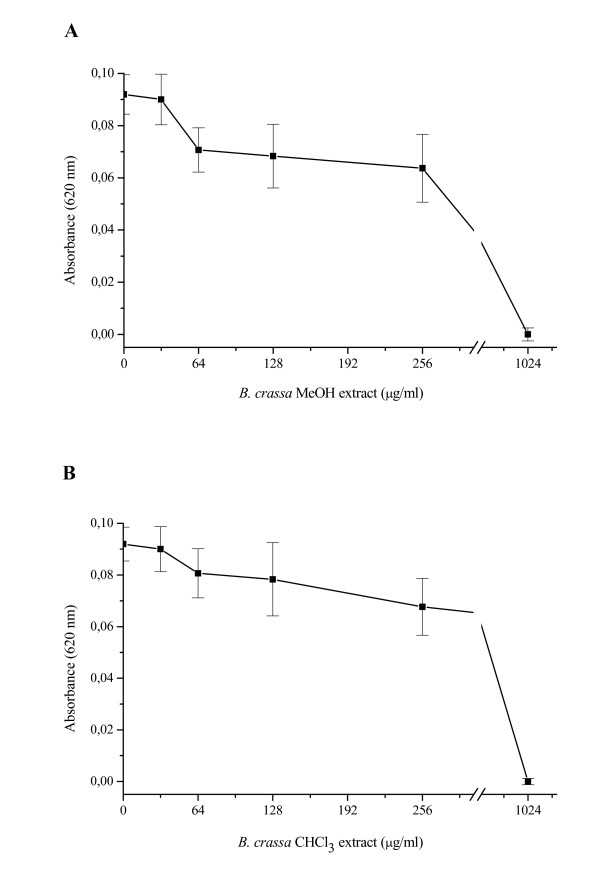
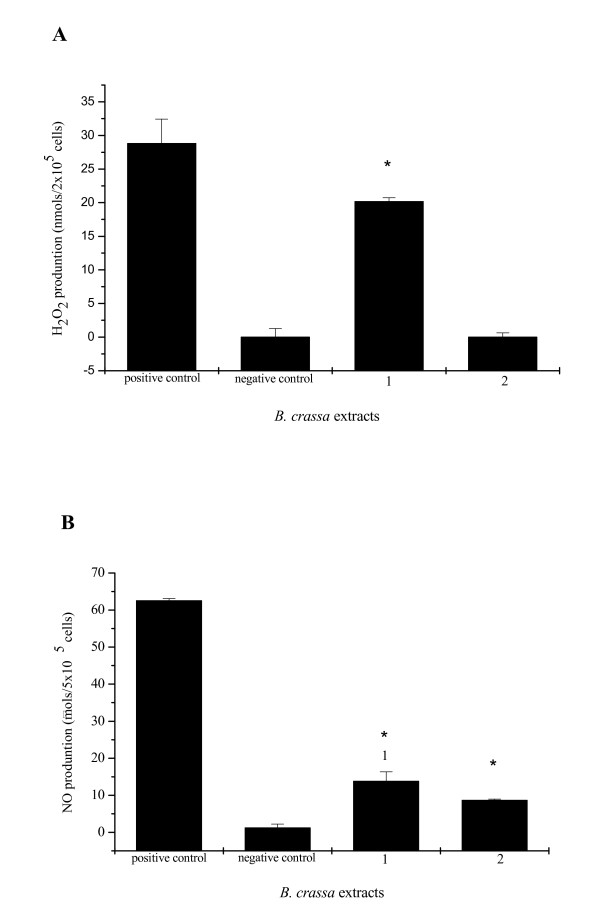
The antibacterial activities of the extracts (MeOH and CHCl3) from B. crassa against H. pylori, using spectrophotometer microdilution assay, are showed in Figure 1. The results demonstrated that both MeOH and CHCl3 extract exhibited anti-H. pylory activity with MIC value of 1024 μg/ml. The data presented in Figure 2 summarize the release of H2O2 and NO by murine peritoneal macrophages. The MeOH extract at concentrations of 280 μg/ml (maximum concentration that did not cause cell death) was able to induce the release of 20.16 ± 0.58 nmol/2 × 105 cells and 13.79 ± 2.58 μmol/5 × 105 cells H2O2 and NO, respectively. The CHCl3 extract at concentration of 200 μg/ml (maximum concentration that did not cause cell death) just induced the production of NO (8.63 ± 0.35 μmols/5 × 105 cells).
Figure 1.
Effect of the MeOH (A) and CHCl3 (B) extracts obtained of Byrsonima crassa on growth of Helicobacter pylori, following incubation for 72 h.
Figure 2.
H2O2 (A) and NO (B) production from peritoneal macrophages stimulated with 280 μg/ml MeOH (1) and 200 μg/ml CHCl3 (2) extracts. Macrophages were exposed to Byrsonima crassa extracts for 1 h (A) and 24 h (B). The results are the mean ± SD of at least three independents experiments carried out in triplicate. *P < 0.05, significantly different from control without stimulating (negative control).
Discussion
Many anti-H. pylori compounds exhibiting a significant inhibitory effect have been identified from plant materials, among them flavonoids, tannins, terpenes, aromatic aldehydes, alcohols, tannins and catechins [18,29-31]. Based on the popular use of B. crassa to treat gastritis and ulcer, we investigated, in vitro, the susceptibility of H. pylori ATCC 43504 (AmxS and MtzR strain) to MeOH and CHCl3 extracts obtained from the leaves of this medicinal plant. Both extracts presented MIC value of 1024 μg/ml. In summary, our results suggest that B. crassa produces secondary metabolites with anti-H. pylori activity. Probably, the antimicrobial activity demonstrated by extracts may be due to the presence of polyphenolic compounds, such as flavonoids, tannins, and terpenoids described in the phytochemical profile of B. crassa [16,32,33], not discarding the possibility of a synergistic effects between substances.
H. pylori infection has been associated with generation of oxygen (ROS) and nitrogen (RNS) reactive species, which leads to oxidative stress in gastric mucosa [22,34,35]. This bacterium induces infiltration and activation of phagocytes, which produce inflammatory mediators, cytokines, ROS and RNS. To avoid the negative effects of ROS, H. pylori, like many other bacteria, produces enzymes involved in ROS scavenging, such as catalase and superoxide dismutase. H. pylori also activates inducible nitric oxide synthase in the gastric mucosa, which is associated with epithelial cell injury and apoptosis [2]. No evidence was found for a role of free radicals in the pathogenesis of gastric mucosal injury in cases unrelated to H. pylori infection [35].
Macrophages are widely distributed in different tissues and play an essential role in the development of the specific and nonspecific immune response. These cells can be activated by a variety of stimuli as bacterial components, cytokines and chemicals. Once activated, macrophages produce and release numerous secretors products including several cytokines, inorganic reactive radicals, reactive oxygen intermediates (ROI) and reactive nitrogen intermediates (RNI) with biological activities [36]. Hydrogen peroxide (H2O2) and nitric oxide (NO) are important in cell signaling and they are effectors molecules for microbicidal and cytotoxic response of macrophages after stimulation [37]. If ROI and RNI may be considered as beneficial intermediates (with respect to its microbicidal and tumoricidal activities), it also can become destructive for the host tissue in certain conditions [38]. NO and reactive oxygen species affect virtually every step of the development of inflammation.
The immunomodulatory activity of several plants compounds has already been described [39]. Davilla elliptica chloroform extract triggered the production of H2O2, NO and tumor necrosis factor-alpha in a dose-dependent manner into cultured macrophages [40] whereas inhibitory effects in H2O2 and NO production by ethyl acetate fraction from Alchornea glandulosa were demonstrated [41]. In particular, those plants that reduce the formation of NO may be beneficial in pathophysiological conditions where excessive production of NO is a contributory factor.
In this study, we demonstrated that extracts of B. crassa induced the production H2O2 and NO. Agents that reduce the formation of NO may be beneficial in H. pylori infections since an excessive production of NO is an agravating factor in this condition. Finally, increased production of free radicals has been demonstrated to occur during the gastrointestinal metabolism of xenobiotics, which may lead to intestinal disorders [42].
Despite of the antiulcerogenic effect exhibited by B. crassa associated with HCl/ethanol induced gastric ulcers [16] and inhibitory activity against H. pylori, an immunostimulatory effect on the liberation of H2O2 and NO by B. crassa leaves was demonstrated. Based in these results, B. crassa can be considered a source of compounds with anti-H. pylori activity, but its use should be done with caution in treatment of the gastritis and peptic ulcers, since the reactive oxygen/nitrogen intermediates are involved in the pathogenesis of gastric mucosal injury induced ulcerogenic agents and H. pylori infections.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MS and WV have been involved in the obtaining the extracts. IZC performed the immunological assays. CB and MSGR carried out the cell viability, antimicrobial experiments and preparation of the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
We thank the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) and to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CAPES) by financial support.
Contributor Information
Cibele Bonacorsi, Email: bonac@terra.com.br.
Maria Stella G Raddi, Email: raddims@fcfar.unesp.br.
Iracilda Z Carlos, Email: carlosiz@fcfar.unesp.br.
Miriam Sannomiya, Email: miriamsa@posgrad.iq.unesp.br.
Wagner Vilegas, Email: vilegasw@iq.unesp.br.
References
- Stege PW, Davicino RC, Veja AE, Casali YA, Correa S, Micalizzi B. Antimicrobial activity of aqueous extracts of Larrea divaricata Cav (jarilla) against Helicobacter pylori. Phytomedicine. 2006;13:724–727. doi: 10.1016/j.phymed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Baldari CT, Lanzavecchia A, Telford JL. Immune subversion by Helicobacter pylori. Trends Immunol. 2005;26:199–207. doi: 10.1016/j.it.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Matthews GM, Butler RN. Cellular mucosal defense during Helicobacter pylori infection: a review of the role of glutathione and oxidative pentose pathway. Helicobacter. 2005;10:298–306. doi: 10.1111/j.1523-5378.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- Montecucco C, Rappuoli R. Living dangerously: how Helicobacter pylori survive in the human stomach. Nat Rev Mol Cell Biol. 2001;2:457–466. doi: 10.1038/35073084. [DOI] [PubMed] [Google Scholar]
- Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. Am J Gastroenterol. 1998;93:2330–2338. doi: 10.1111/j.1572-0241.1998.00684.x. [DOI] [PubMed] [Google Scholar]
- Hoffman JS. Pharmacological therapy of Helicobacter pylori infection. Semin Gastrointest Dis. 1997;8:156–163. [PubMed] [Google Scholar]
- Glupczynski Y. Antimicrobial resistance in Helicobacter pylori: a global overview. Acta Gastro-Ent Belg. 1998;61:357–366. [PubMed] [Google Scholar]
- Babincová M, Schronerová K, Sourivong P. Antiulcer activity of water extract of Scoparia dulcis. Fitoterapia. 2008;79:587–588. doi: 10.1016/j.fitote.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Falcão HS, Mariath IR, Diniz MF, Batista LM, Barbosa-Filho JM. Plants of the American continent with antiulcer activity. Phytomedicine. 2008;15:132–46. doi: 10.1016/j.phymed.2007.07.057. [DOI] [PubMed] [Google Scholar]
- Hiruma-Lima CA, Calvo TR, Rodrigues CM, Andrade FD, Souza-Brito ARM. Antiulcerogenic activity of Alchornea castaneaefolia: effects on somatostatin, gastrin and prostaglandin. J Ethnopharmacol. 2006;104:215–224. doi: 10.1016/j.jep.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Donatini RS, Diaz IE, Yoshida M, Bacchi EM, Kato ET. Evaluation of gastroprotective activity of Plinia edulis (Vell.) Sobral (Myrtaceae) leaves in rats. J Ethnopharmacol. 2008;118:527–529. doi: 10.1016/j.jep.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Martínez-Vásquez M, González-Esquinca AR, Luna LC, Moreno Gutiérrez MN, Garcia-Argáez AN. Antimicrobial activity of Byrsonima crassifolia (L.) H.B.K. J Ethnopharmacol. 1999;66:79–82. doi: 10.1016/S0378-8741(98)00155-X. [DOI] [PubMed] [Google Scholar]
- Sannomiya M, Cardoso CRP, Figueiredo ME, Rodrigues CM, dos Santos LC, dos Santos FV, Serpeloni JM, Cólus IMS, Vilegas W, Varanda EA. Mutagenic evaluation and chemical investigation of Byrsonima intermedia A. Juss. leaf extracts. J Ethnopharmacol. 2007;112:319–326. doi: 10.1016/j.jep.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Joly AB. Botânica: Introdução à Taxonomia Vegetal. Companhia Ed Nacional, São Paulo; 1998. p. 808. [Google Scholar]
- Silva SR, Silva AP, Munhoz CB, Silva MC, Jr, Medeiros MB. Guia de Plantas do Cerrado utilizadas na Chapada dos Veadeiros. Prática Gráfica e Editora Ltda, Brasília; 2001. p. 132. [Google Scholar]
- Sannomiya M, Fonseca VB, da Silva MA, Rocha LR, dos Santos LC, Hiruma-Lima CA, Souza-Brito ARM, Vilegas W. Flavonoids and antiulcerogenic activity from Byrsonima crassa leaves extracts. J Ethnopharmacol. 2005;97:1–6. doi: 10.1016/j.jep.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Iwai K, Onodera A, Matsue H. Antioxidant activity and inhibitory effect of Gamazumi (Viburnum dilatatum Thumb.) on oxidative damage induced by water immersion restraint stress in rat. Int J Food Sci Nutr. 2001;52:443–451. doi: 10.1080/09637480120078339. [DOI] [PubMed] [Google Scholar]
- Funatogawa K, Hayashi S, Shimomura H, Yoshida T, Hatano T, Ito H, Hirai Y. Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori. Microbiol Immunol. 2004;48:251–261. doi: 10.1111/j.1348-0421.2004.tb03521.x. [DOI] [PubMed] [Google Scholar]
- Menghini L, Epifano F, Leporini L, Pagiotti R, Tirillini B. Phytochemical investigation on leaf extract of Cordia salicifolia Cham. J Med Food. 2008;11:193–194. doi: 10.1089/jmf.2007.583. [DOI] [PubMed] [Google Scholar]
- Ndipa RN, Tarkanga AEM, Mbullaha SM, Lumab HN, Malongueb A, Ndipa LN, Nyongbelac K, Wirmumd C, Efangec SMN. In vitro anti-Helicobacter pylori activity of extracts of selected medicinal plants from North West Cameroon. J Ethnopharmacol. 2007;114:452–7. doi: 10.1016/j.jep.2007.08.037. [DOI] [PubMed] [Google Scholar]
- Shikov AN, Pozharitskaya ON, Makarov VG, Kvetnaya AS. Antibacterial activity of Chamomilla recutita oil extract against Helicobacter pylori. Phytother Res. 2008;22:252–253. doi: 10.1002/ptr.2243. [DOI] [PubMed] [Google Scholar]
- Arend A, Loime L, Roosaar P, Soom M, Lõivukene K, Sepp E, Aunapuu M, Zilmer K, Selstam G, Zilmer M. Helicobacter pylori substantially increases oxidative stress in indomethacin-exposed rat gastric mucosa. Medicina (Kaunas) 2005;41:343–347. [PubMed] [Google Scholar]
- Bagchi D, McGinn TR, Ye X, Bagchi M, Krohn RL, Chatterjee A, Stohs SJ. Helicobacter pylori-induced oxidative stress and DNA damage in a primary culture of human gastric mucosal cells. Dig Dis Sci. 2002;47:1405–1412. doi: 10.1023/A:1015399204069. [DOI] [PubMed] [Google Scholar]
- Ernst P. Review article: the role of inflammation in the pathogenesis of gastric cancer. Aliment Pharmacol Ther. 1999;13:13–18. doi: 10.1046/j.1365-2036.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- Eloff JN. Which extractant should be used for the screening and isolation of antimicrobial components from plants. J Ethnopharmacol. 1998;60:1–8. doi: 10.1016/S0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- Borenfreund E, Puerner JA. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985;24:119–124. doi: 10.1016/0378-4274(85)90046-3. [DOI] [PubMed] [Google Scholar]
- Pick E, Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme immunoassay reader. J Immunol Methods. 1981;46:211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogorwki J, Skipper PL, Wisnok JS, Tannenbowm SR. Analysis of nitrate, nitrite and (15N) nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- Fukai T, Marumo A, Kaitou K, Kanda T, Tereda S, Nomura T. Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002;71:1449–1463. doi: 10.1016/S0024-3205(02)01864-7. [DOI] [PubMed] [Google Scholar]
- Nostro A, Cellini L, Di Bartolomeo S, Di Campli E, Grande R, Cannatelli MA, Marzio L, Alonzo V. Antibacterial effect of plants extracts against Helicobacter pylori. Phytother Res. 2005;19:198–202. doi: 10.1002/ptr.1640. [DOI] [PubMed] [Google Scholar]
- Shin JE, Kim JM, Bae EA, Hyun YJ, Kim DH. In vitro inhibitory effect of flavonoids on growth, infection and vacuolation of Helicobacter pylori. Planta Med. 2005;71:197–201. doi: 10.1055/s-2005-837816. [DOI] [PubMed] [Google Scholar]
- Arantes VP, Sato DN, Vilegas W, Santos LC, Leite CQF. Plantas do cerrado brasileiro com atividade contra Mycobacterium fortuitum. Rev Ciênc Farm Básica Apl. 2005;26:195–198. [Google Scholar]
- Cardoso CR, de Syllos Cólus IM, Bernardi CC, Sannomiya M, Vilegas W, Varanda EA. Mutagenic activity promoted by amentoflavone and methanolic extract of Byrsonima crassa Niedenzu. Toxicol. 2006;225:55–63. doi: 10.1016/j.tox.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Allen LH, Beecher BR, Lynch JT, Rohner V, Wittine LM. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J Immunol. 2005;174:3658–3667. doi: 10.4049/jimmunol.174.6.3658. [DOI] [PubMed] [Google Scholar]
- Davies GR, Simmonds NJ, Rampton DS. Helicobacter pylori stimulates antral mucosal reactive oxygen metabolite production in vivo. Gut. 1994;35:179–185. doi: 10.1136/gut.35.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman HJ, Torres M. Redox signaling in macrophages. Mol Aspects Med. 2001;22:189–216. doi: 10.1016/S0098-2997(01)00010-3. [DOI] [PubMed] [Google Scholar]
- Kayser O, Kolodziej H, Kiderlen AF. Immunomodulatory principles of Pelargonium sidoides. Phytother Res. 2000;15:122–126. doi: 10.1002/ptr.785. [DOI] [PubMed] [Google Scholar]
- McBride AG, Borutaité V, Brown GC. Superoxide dismutase and hydrogen peroxide cause rapid nitric oxide breakdown, peroxynitrite production and subsequent cell death. Biochim Biophys Acta. 1999;1454:275–88. doi: 10.1016/s0925-4439(99)00046-0. [DOI] [PubMed] [Google Scholar]
- Moreira RRD, Carlos IZ, Vilegas W. Release of intermediate reactive hydrogen peroxide by macrophage cells actived by natural products. Biol Pharm Bull. 2001;24:201–204. doi: 10.1248/bpb.24.201. [DOI] [PubMed] [Google Scholar]
- Mascia Lopes FC, Polesi Placeres MC, Jordão CM, Jr, Higuchi CT, Rinaldo D, Vilegas W, Fujimura Leite CQ, Carlos IZ. Immunological and microbiological activity of Davilla elliptica St. Hill. (Dilleniaceae) against Mycobacterium tuberculosis. Mem Inst Oswaldo Cruz. 2007;102:769–72. [PubMed] [Google Scholar]
- Lopes FCM, Calvo TR, Vilegas W, Carlos IZ. Inhibition of hydrogen peroxide, nitric oxide and TNF- Alchornea glandulosa. 2005;28:1726–1730. doi: 10.1248/bpb.28.1726. [DOI] [PubMed] [Google Scholar]
- Mansbach CM, Rosen GM, Rahn CA, Strauss KE. Detection of free radicals as a consequence of rat intestinal cellular drug metabolism. Biochim Biophys Acta. 1986;888:1–9. doi: 10.1016/0167-4889(86)90063-7. [DOI] [PubMed] [Google Scholar]