Abstract
The purpose of this study is to examine the intracellular distribution of collagen types I, III and V in tenocytes using triple-label immunofluorescence staining technique in high-density tenocyte culture on Filter Well Inserts (FWI). The tenocytes were incubated for 4 weeks under monolayer conditions and for 3 weeks on FWI. At the end of the third week of high-density culture, we observed tenocyte aggregation followed by macromass cluster formation. Immunofluorescence labeling with anti-collagen type I antibody revealed that the presence of collagen type I was mostly around the nucleus. Type III collagen was more diffused in the cytoplasm. Type V collagen was detected in fibrillar and vesicular forms in the cytoplasm. We conclude that, the high-density culture on FWI is an appropriate method for the production of tenocytes without loosing specialized processes such as the synthesis of different collagen molecules. We consider that the high-density culture system is suitable for in vitro applications which affect tendon biology and will improve our understanding of the biological behavior of tenocytes in view of adequate matrix structure synthesis. Such high-density cultures may serve as a model system to provide sufficient quantities of tenocytes to prepare tenocyte-polymer constructs for tissue engineering applications in tendon repair.
Keywords: Cell culture, Cell-material interactions, Collagen, Immunocytochemistry, Tenocyte
Introduction
In recent years, in vitro cell production with different tissue origins such as heart muscle, nervous and connective tissues is popular in regeneration therapies of various diseases. Among these, implantation therapies of tenocytes used in the regeneration of tendon injuries are quite important. As a result of repeated movement and degeneration which occur in the course of time, tendons are functionally open to traumas which can come into existence in both acute and chronic ways (Lin et al. 2006). In order to solve this problem, several tissue engineers have produced tenocytes which are buried into biopolymers and autologous mesenchymal cells originating from different tissues such as bone marrow (Cao et al. 1994; Awad et al. 1995; Koob et al. 2001).
Tendon is the structure which binds skeletal muscles to bones, functions to transmit the force on muscle in one direction and works as a shock absorbent. Tendon dry weight is composed of 75–90% various collagen molecules, 0.2–5% proteoglycans, 1–2% elastin. The remainder is fibronectin (Elliott 1965; Gillard et al. 1977; Okuda et al. 1987). Type I collagen is the main component of tendon; but there are also type III, type V collagens and proteoglycans in the structure. The packaging property of variable collagen fibrils as heterotypic fibrils supplies different amounts of resistance in various tissues (Birk 2001). According to Linsenmayer et al. (1993) and Fleischmayer et al. (1990), during the fibrillogenesis in tendon tissue, heterotypic fibril formation by type V/type I collagen and type III/type I collagen has a role on the regulation of fibril diameter. In tendon tissue, type V collagen fibrils form a core structure for type I collagen fibrils to bind and to form stable and more compact fibril packages in diameter with type III collagen.
Tendon cells which are known as tenocytes are responsible for the production of extracellular components that consist of collagen, proteoglycans, fibronectin and elastic fibrils (Nakagawa et al. 1994; Scott 1984). There are several studies which describe the culture of tendon cells in different species. Bernard-Beaubois et al. (1997), Schwarz et al. (1976), and Schulze-Tanzil et al. (2004) have described that tenocytes are capable of synthesizing type I collagen in high-density culture conditions during the first 2 weeks of cultivation. Tenocytes synthesize mainly type I, type III, type V (Hong et al. 1979) and type VI collagens (Bruns 1984) and proteoglycans (Scott et al. 1981; Vogel et al. 1984). These cells contribute to collagen production with their specialized cell surfaces (Trelstad 1982) and form three dimensional matrices under in vitro conditions (Evans and Trail 1998). Nevertheless, Bernard and Beaubois et al. (1997) have shown that the structure formed by these cells is insufficient for maintaining the specialized characters of tenocytes, while monolayer cultures are not appropriate to imitate in vivo matrix. During the long-term cultivation of tenocytes, the main problem that is encounted is the reduced synthesis of type I, type III and type V collagens (Schwarz et al. 1976; Birk and Trelstad 1986). In order to address these issues, Schulze-Tanzil et al. (2004) have developed the high-density three dimensional culture system for the cultivation of phenotypically authentic tenocytes in vitro. Millicell Filter Well Inserts are valuable cell biology tools designed to improve cell attachment, growth and differentiation over traditional plastic cell culture plates. Filter Well Inserts promote a more natural cell growth by allowing media access from both the apical and basolateral sides of the membrane. This allows cells to mimic in vivo conditions closely. Millicell inserts provide high cell viability—as long as 40 days—and superior study of three dimensional opportunity for the cell studies with excellent trans-membrane oxygen transport which are available in mixed cellulose esters and polycarbonate membrane. Inserts have a unique design which allows easier basolateral access to medium compared to other hanging inserts with less risk of contamination. Inserts are available in five different pore sizes and three different diameters, including 1 μm pore size that is optically transparent for better visualization by microscopy.
In this study, the intracellular arrangement of collagen types I, III and V was examined by triple-immunofluorescence staining technique in extended high-density cultured and macromass cluster forming tenocytes on Filter Well Inserts.
Materials and methods
Antibodies and immunofluorescence kits
Monoclonal mouse anti-rat IgG type-I collagen (NB600-448), monoclonal mouse anti-rat IgG type-III collagen which binds an epitope near the N-terminal of type III collagen (MAB3392) and monoclonal mouse anti-rat IgM type-V collagen (MAB3302) antibodies were purchased from Chemicon International. Biotinylated secondary goat anti-mouse antibodies, fluorochroms, M.O.M.™ Kit—Fluorescent (FMK-2201), M.O.M.™ Kit—Basic (BMK-2202), Avidin/Biotin Blockage Kit (SP-2001), VECTASHIELD® Hard Set Mounting Medium (H-1400), AMCA Avidin D (A-2008) and Texas Red Avidin D (A-2006) were purchased from Vector Laboratories, Inc. for immunofluorescence staining.
Growth medium and additive solutions
DMEM/F12 (Dulbecco’s Modified Eagle’s Medium/F12 Nutrient Mixture) (SID8437), FBS (Fetal Bovine Serum) (SIF6178), L-ascorbic acid (SIA4403) [100 mg powder form which was used in 25 μg/mL concentration], glutamine–penicillin–streptomycin solution (SIG6784), 0.25% Trypsin-EDTA solution (SIT4049) and MEM amino acids solution 50× (SIM5550) were purchased from Sigma–Aldrich, Inc.
Other materials
Six multiwell plates (3516) were purchased from Costar® and Millicell® Filterwell Inserts (PIHP03050) were purchased from Millipore.
Monolayer tenocyte culture
Tenocytes needed to set up the monolayer culture were isolated from Achilles tendon of three neonatal (20 days old) common laboratory rats (Rattus norvecigus) by tissue explant technique under sterile conditions. All experimental procedures and animals’ use were approved by the Ethics Committee of Hacettepe University. We excised the peritendineum, removed the remaining tendon tissue with sterile techniques and rinsed in physiological saline solution. A few pieces of fresh tendon (about 1–2 mm) were placed in six well plates and cultured in DMEM/F12 growth medium containing 10% FBS, 2.5 μg/100 mL L-ascorbic acid, 1 mL/100 mL glutamine-penicillin-streptomycin solution and 1 mL/100 mL amino acid solution. After 1 week, tenocytes were continuously migrating from the adhered tendon pieces. After obtaining confluency in monolayer cultures, plate surfaces were washed with 0.25% Trypsin-EDTA solution for passaging. Tenocytes were passaged six times, intermittently on 3 days to gain sufficient amount of cells for the preparation of high-density culture.
High-density tenocyte culture
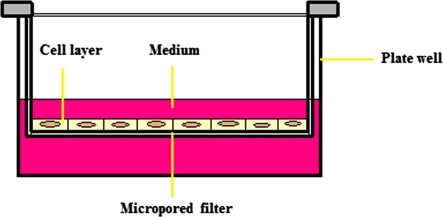
After 4 weeks in monolayer culture, cells belonging to each separate well were counted. According to Millicell® and Freshney’s (2001) advices, we decided to seed 5 × 105 cells on each Filter Well Insert (FWI) to allow the recombination of cells at very high, tissue-like densities, with ready access to medium and gas exchange, but in a multireplicate form (Fig. 1). Before placing the FWIs in plates, 2.0 mL of medium was added in all wells. After placing the FWIs, 1.0 mL of 5 × 105 cells were seeded above the membranes and an additive 1.0 mL of medium was added above the cells in order to make inside and outside medium volumes equal. Cells were incubated for 3 weeks in the high-density culture conditions at the medium-air interface in multiwell dishes as described in detail by Zimmermann et al. (1992) and Shulze-Tanzil et al. (2004). Cultures were grown at 37 °C in a humidified atmosphere with 5% CO2 and medium was refreshed every 2 days.
Fig. 1.
Illustration of the FWI placed in a plate well
Triple-immunofluorescence staining
The tenocytes were fixed with pure acetone for 10 min. Then, washed a few minutes with phosphate buffered saline (PBS), pH 7.5. For second and third antibodies, Avidin/Biotin blocking was performed for 15 min. Cells were incubated for 20 min with buffer containing 5% normal serum for protein blockage. Primary antibodies for anti-type I (1:2000), anti-type III (1:500), anti-type V collagen molecules (1:500) and secondary antibodies were diluted in 5% normal serum. Cells were incubated for 1 h with primary antibodies, 10 min with secondary antibodies and then washed a few times with PBS and incubated with the appropriate fluorochrome molecule until optimal color developed. AMCA was used for type I, FITC was used for type III and Texas Red was used for type V collagen molecule’s staining. After washing with PBS, slides were mounted with 25 μL VECTASHIELD® Hard Set Mounting Medium. Digital images were captured with AXIO Vision.4B software.
Results
Monolayer culture and light microscopy
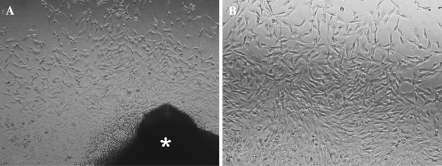
After 5 days in culture, tenocytes started to migrate from the tendon tissue, adhered on the base of the wells and formed a monolayer culture (Fig. 2a). At the end of the 10th day, the cells became confluent and exhibited long cytoplasmic extensions as typical tendon fibroblasts. These extensions seemed to facilitate the formation of contact with other neighboring tenocytes. The cells were bipolar, spindle shaped with mostly round-oval nuclei (Fig. 2b).
Fig. 2.
Tenocytes cultured 1 week in monolayer culture a Cells migrating from the tendon tissue (*) on 5th day (×10) b Confluent monolayer tenocytes with spindle shape (×20)
Formation of macromass clusters in high-density cultured tenocytes
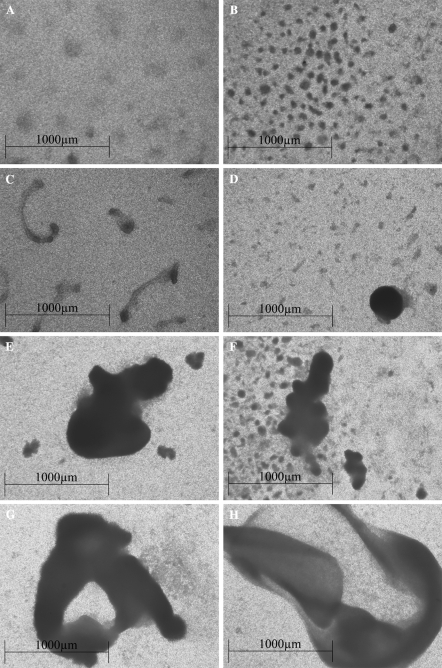
The tenocytes were incubated for 3 weeks in high-density culture conditions. On the first day of high-density culture, cells on Filter Well Inserts (FWI) appeared with typical tenocyte morphology with direct cell to cell contact with other neighboring cells. Over the following days intercellular spaces widened and tenocytes started to form aggregates of cells (Fig. 3a). At the end of the first week, these aggregates became clearer with more cells attaching to the clusters (Fig. 3b). In the second week, clusters started to connect with each other, forming larger macromass clusters (Fig. 3c). At the end of the third week, macromass clusters became 1–2 mm in size by the aggregation of other neighboring clusters which were large enough to be seen with naked-eye (Fig. 3d–h).
Fig. 3.
Macromass cluster forming tenocytes which were cultured 3 weeks in high-density culture (×30). a Tenocytes forming aggregates. b At the end of the first week, these aggregates became clearer with more cells attaching the clusters. c Clusters connecting with each other and forming macromass clusters. d–h The appearance of macromass clusters at the end of third week
Intracellular collagen characterization in high-density cultured tenocytes
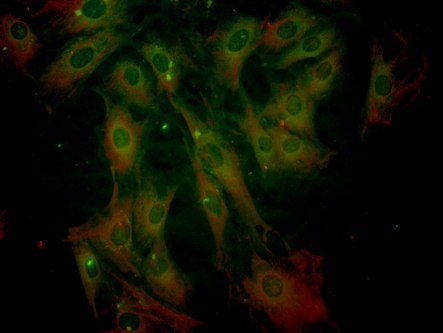
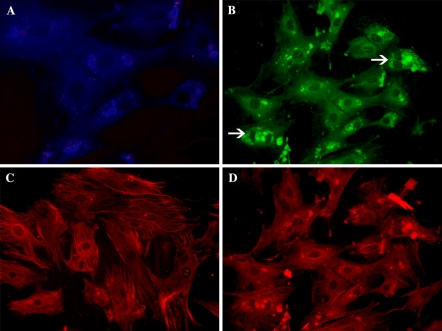
Immunofluorescence microscopy of antibody-labeled tenocytes which were incubated for 7 weeks (4 weeks in monolayer and 3 weeks in high-density culture) showed positive staining for the typical extracellular matrix components of tendon such as, type I, type III and type V collagen molecules (Fig. 4). Type I collagen was stained in blue color with AMCA, type III collagen was stained in green color with FITC and type V collagen was stained in red color with Texas Red fluorochrome.
Fig. 4.
Immunofluorescence staining of tenocytes which were incubated 7 weeks (4 weeks in monolayer and 3 weeks in the high-density culture) showed positive staining for type I (blue color with AMCA), type III (green color with FITC) and type V collagen molecules (red color with Texas Red fluorochrome) (×120)
Immunofluorescence labeling with anti-collagen type I antibody revealed that the presence of collagen type I was mostly around the nucleus (Fig. 5a). Labeling with anti-collagen type III antibody revealed that the presence of collagen type III was more diffused in the cytoplasm when compared with type I and type V collagens (Fig. 5b). Type III collagen vesicles belonging to tenocytes which completed karyokinesis but didn’t complete cytokinesis showed more dense and clear staining (Fig. 5b, white arrows). Labeling with anti-collagen type V antibody revealed that the presence of collagen type V was in both fibrillar (Fig. 5c) and vesicular forms (Fig. 5d) diffused in cytoplasm. In smaller tenocytes, type V collagen showed more dense and bright staining.
Fig. 5.
Tenocytes cultured for 3 weeks in the high-density culture. a Labeled with anti-collagen type I (×80) b anti-collagen type III (×80) and c anti-collagen type V (fibrillar) (×80) and d anti-collagen type V (vesicular) antibodies (×80) examined by immunofluorescence microscopy
Discussion
As a result of repeated movement and degeneration which occurs in the course of time, tendons are functionally open to traumas which can come into existence in both acute and chronic ways (Lin et al. 2006). In recent time, trauma which occurs in sportsman is very common. For this reason, the investigation of the constructive effects of in vitro produced tenocytes on the regeneration is inevitable (Birk and Trelstad 1986).
As tenocytes have low mitotic activity (Ahmed et al. 1998) and lack of vascularization, regeneration in the damaged area occurs very slowly (Woo et al. 1999; Möller et al. 2000). According to this problem, several tissue engineers have produced tenocytes which are buried into biopolymers and autologous mesenchymal cells originating from different tissues such as bone marrow (Cao et al. 1994; Awad et al. 1995; Koob et al. 2001).
The success of transplantation therapies after tendon traumas correlates with the capacity of transplanted tenocytes to produce a tendon matrix containing different collagens. The main problem that is encountered during long-term monolayer cultivation of these cells is the reduced synthesis of type I, type III and type V collagens (Schwarz et al. 1976; Birk and Trelstad 1986). Bernard-Beaubois et al. (1997) have reported that the extended monolayer cultivation of tenocytes lead to decreased levels of collagen type I. According to some researchers tenocytes can form three dimensional matrix under in vitro conditions (Evans and Trail 1998). Nevertheless, recent studies have shown that the structure formed by these cells is insufficient for maintaining specialized processes of tenocytes and the monolayer cultures are not appropriate to imitate in vivo matrix under in vitro conditions (Bernard-Beaubois et al. 1997). Therefore, the necessity of high density culture systems has been suggested by Schulze-Tanzil et al. (2004) These researchers have reported that, by imitating in vivo conditions in high-density culture, human tenocytes were able to continue their differentiated characters such as collagen synthesis up to 2 weeks. Based on these findings, we have modified Schulze-Tanzil and colleagues’ (2004) high-density culture method by using filter well inserts which supplied a tissue-like environment for tenocytes. The present study demonstrates that, in the high-density culture conditions, tenocytes have formed aggregates which further developed into larger macromass clusters. Tenocytes belonging to these aggregates have continued synthesizing different collagen molecules throughout 7 weeks in culture. According to the widening of intercellular spaces during aggregate formation, we can assume that the loss of cell–cell and cell–matrix interactions may be a reason for the loss of collagen synthesis in extended monolayer culture.
Antibody-labeled tenocytes which were incubated for 3 weeks in high-density culture showed positive staining for collagen molecules such as type I, type III and type V. Our staining results indicate the synthesis of collagen molecules by tenocytes, but does not give any information about the amount of synthesized collagen. In order to obtain these amounts, further studies including ELISA tests should be done.
In our study, we detected intracellular type I, type III and type V collagen distribution in tenocyte cytoplasm. Bernard-Beaubois et al. (1997) have determined that type I collagen was localized preferentially around the nuclei. All of our staining results showed that the distribution of type I collagen vesicles were only around nuclei. These results are similar with the Bernard-Beaubois et al.’s (1997) findings. However, in Fig. 3, positive stainings for type I collagen cannot be identified. The main reason of this situation is the staining of type III collagen with FITC fluorochrome around the same perinuclear area as type I collagen. The green color reflected by FITC is more dominant to the blue color reflected by AMCA fluorochrome. Type III procollagen/collagen staining was more diffused in the cytoplasm and also more close to cell membrane when compared with type I collagen. Gey et al. (1974) have studied using fibroblast cultures and reported that type I collagen is more diffused in perinuclear region in proportion to type III collagen, when type III collagen is more diffused in intracellular and extracellular areas. Our results are in accordance with Gey et al.’s (1974) study. Type V procollagen/collagen was found as fibrillar and vesicular form. When all staining results were compared, it is evident that the molecule that gives the brightest staining is type V procollagen/collagen. According to Birk (2001), type V collagen is needed in order to form the heterotypic fibril structure with type I collagen in tendon. This may be the reason of over-staining determined in our tenocytes. It was not possible to discuss these results in more detail with other researchers’ findings, because there was insufficiency of sources about type V collagen distribution in tenocyte cytoplasm.
Conclusions
We conclude that, high density culture system with filter well inserts is an appropriate method for the production of tenocytes under in vitro conditions and preventing the loss of specialized processes such as collagen production and cellular aggregation in order to form tissue-like clusters. According to our results, under certain conditions, tenocytes were still synthesizing different collagen molecules at the end of the 7th week. In our study, distribution of type V collagen in tenocyte cytoplasm was defined in detail for the first time. We consider that, this culture system is useful for in vitro applications effecting tendon biology and production of vital tenocytes. As a result, we conclude that the usage of filter well inserts for obtaining high-density cultures is important for the development of further studies that will improve our understanding of the biological behavior of tenocytes in view of adequate matrix structure synthesis in high density culture. Such high-density culture might serve as a model system to provide sufficient quantities of tenocytes to prepare tenocyte-polymer constructs for tissue engineering applications in tendon repairing. Macromass clusters can also be removed from FWI surface with forceps or cell scrapper. This methodology prevents any damage that can occur during mechanical and enzymatic removal of cells and the cell clusters can be directly transferred to the damaged application sites. Therefore, our study can be the directive step for this approach.
Acknowledgments
This study was partially funded by Hacettepe University Scientific Research Unit (project number is 06 003 101 006), and it has been submitted to Hacettepe University as the MSc thesis of Cansın Yaylalı. The tissue culture and fluorescent microscopy studies were conducted at the Central Laboratory of Ankara University Biotechnology Institute.
Abbreviations
- DMEM
Dulbecco’s Modified Eagle’s Medium
- FBS
Fetal Bovine Serum
- FWI
Filter Well Insert
- PBS
Phosphate Buffered Saline
References
- Ahmed IM, Lagopoulos M, McConnell P, Soames RW, Sefton GK (1998) Blood supply of the Achilles tendon. J Orthop Res 16:591–596. doi:10.1002/jor.1100160511 [DOI] [PubMed]
- Awad HA, Butler DL, Boivin GP, Smith FN, Malaviya P, Huibregtse B, Caplan AI (1995) Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng 5:267–277. doi:10.1089/ten.1999.5.267 [DOI] [PubMed]
- Bernard-Beaubois K, Hecquet C, Houcine O, Hayem G, Adolphe M (1997) Culture and characterization of juvenile rabbit tenocytes. Cell Biol Toxicol 13:103–113. doi:10.1023/B:CBTO.0000010395.51944.2a [DOI] [PubMed]
- Birk DE (2001) Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron 32:223–237. doi:10.1016/S0968-4328(00)00043-3 [DOI] [PubMed]
- Birk DE, Trelstad RL (1986) Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J Cell Biol 103:231–240. doi:10.1083/jcb.103.1.231 [DOI] [PMC free article] [PubMed]
- Bruns RR (1984) Beaded filaments and long-spacing fibrils: relation to type VI collagen. J Ultrastruct 89:136–145. doi:10.1016/S0022-5320(84)80010-6 [DOI] [PubMed]
- Cao Y, Vacanti JP, Ma X, Paige KT, Upton J, Chowanski Z, Schloo B, Langer R, Vacanti CA (1994) Generation of neo-tendon using synthetic polymers seeded with tenocytes. Transplant Proc 26:3390–3392 [PubMed]
- Elliott DH (1965) Structure and function of mammalian tendon. Biol Rev Camb Philos Soc 40:392–421. doi:10.1111/j.1469-185X.1965.tb00808.x [DOI] [PubMed]
- Evans CE, Trail IA (1998) Fibroblast-like cells from tendons differ from skin fibroblasts in their ability to form three-dimensional structures in vitro. J Hand Surgery 23:633–641 [DOI] [PubMed]
- Fleischmayer R, Perlish JS, Burgeson RE, Shaikh-Bahai F, Timpl R (1990) Type I and type III collagen interactions during fibrillogenesis. Ann N Y Acad Sci 580:161–175. doi:10.1111/j.1749-6632.1990.tb17927.x [DOI] [PubMed]
- Freshney RI (2001) Culture of animal cells: a manual of basic techniques, 4th edn. Wiley-Liss, Canada
- Gey GO, Svotelis M, Foard M, Bang FB (1974) Long-term growth of chicken fibroblasts on a collagen substrate. Exp Cell Res 84:63–71 [DOI] [PubMed]
- Gillard GC, Merrilees MJ, Bell-Booth PG, Reilly HC, Flint MH (1977) The proteoglycan content and the axial periodicity of collagen in tendon. Biochem J 163:145–151 [DOI] [PMC free article] [PubMed]
- Hong BS, Davison PF, Cannon DJ (1979) Isolation and characterization of distinct type of collagen from bovine fetal membranes and other tissues. Biochemistry 18:4278–4282. doi:10.1021/bi00587a003 [DOI] [PubMed]
- Koob TJ, Willis TA, Qiu YS, Hernandez DJ (2001) Biocompatibility of NDGA-polymerized collagen fibers. II. Attachment, proliferation, and migration of tendon fibroblasts in vitro. J Biomed Mater Res 56:40–48. doi:10.1002/1097-4636(200107)56:1<40::AID-JBM1066>3.0.CO;2-I [DOI] [PubMed]
- Lin SJ, Lo W, Tan HY, Chan JY, Chen WL, Wang SH, Sun Y, Lin WC, Chen JS, Hsu CJ, Tjiu JW, Yu HS, Jee SH, Dong CY (2006) Prediction of heat-induced collagen shrinkage by use of second harmonic generation microscopy. J Biomed Opt 11:34020. doi:10.1117/1.2209959 [DOI] [PubMed]
- Linsenmayer TF, Gibney E, Igoe F (1993) Type V collagen: molecular structure and fibrillar organization of the chicken alpha 1(V) NH2-termianl domain, a putative regulator of corneal fibrillogenesis. J Cell Biol 121:1181. doi:10.1083/jcb.121.5.1181 [DOI] [PMC free article] [PubMed]
- Möller HD, Evans CH, Maffulli N (2000) Current aspects of tendon healing. Orthopad 29:182–187 [DOI] [PubMed]
- Nakagawa Y, Majima T, Nagashima K (1994) Effect of ageing on ultrastructure of slow and fast skeletal muscle tendon in rabbit Achilles tendons. Acta Physiol Scand 152:307–313. doi:10.1111/j.1748-1716.1994.tb09810.x [DOI] [PubMed]
- Okuda Y, Gorski JP, An KN, Amadio PC (1987) Biochemical, histological, and biomechanical analysis of canine tendon. J Orthop Res 5:60–68. doi:10.1002/jor.1100050109 [DOI] [PubMed]
- Schulze-Tanzil G, Mobasheri A, Clegg PD, Sendzik J, John T, Shakibaei M (2004) Cultivation of human tenocytes in high-density culture. Histochem Cell Biol 122:219–228. doi:10.1007/s00418-004-0694-9 [DOI] [PubMed]
- Schwarz R, Colarusso L, Doty P (1976) Maintenance of differentiation in primary cultures of avian tendon cells. Exp Cell Res 102:63–71. doi:10.1016/0014-4827(76)90299-8 [DOI] [PubMed]
- Scott JE (1984) The periphery of the developing collagen fibril. Biochem J 218:229–233 [DOI] [PMC free article] [PubMed]
- Scott JE, Orford CR, Huges EW (1981) Proteoglycan-collagen arrangements in rat tail tendon. Biochem J 195:573–581 [DOI] [PMC free article] [PubMed]
- Trelstad RL (1982) Multistep assembly of type I collagen fibrils. Cell 28:197–198. doi:10.1016/0092-8674(82)90334-8 [DOI] [PubMed]
- Vogel KG, Paulsson M, Heinegard D (1984) Specific inhibition of type I and II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J 223:587–597 [DOI] [PMC free article] [PubMed]
- Woo SL, Hildebrand K, Watanabe N, Fenwick JA, Papageorgion CD, Wang JH (1999) Tissue engineering of ligament and tendon healing. Clin Orthop Relat Res 367:S312–S323. doi:10.1097/00003086-199910001-00030 [DOI] [PubMed]
- Zimmermann B, Shröter-Kermani C, Shakibaei M, Merker H-J (1992) Chondrogenesis, cartilage maturation and transformation of chondrocytes in high-density culture of mouse limb bud mesodermal cells. Eur Arch Biol 103:93–111