FIG. 2. Structure of the AphA and MarR dimers.
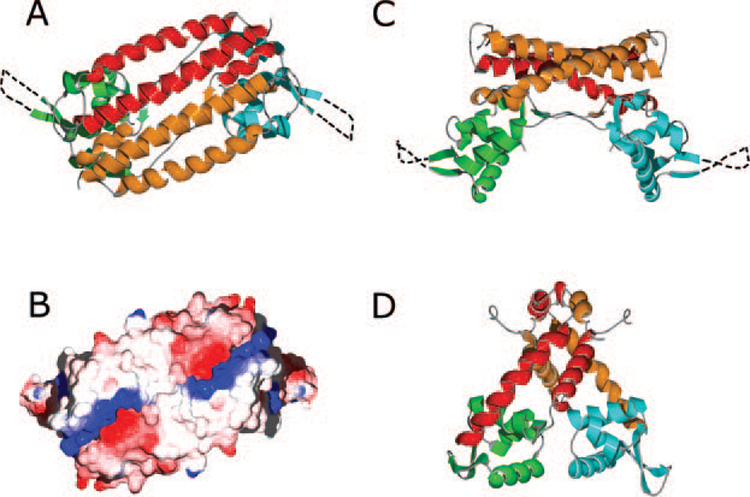
A, the AphA dimer viewed along the crystallographic two-fold axis. In this top view, the DNA binding face is underneath the dimer. For one molecule, the N-terminal DNA binding domain is green, and the C-terminal dimerization domain is red. For the second molecule, the DNA and dimerization domains are blue and orange, respectively. Unresolved residues in the wing motif are shown as a dashed line. B, electrostatic surface potential of the AphA dimer, oriented as in A. The complementary positive (blue) and negative (red) charge clusters that stabilize the dimer interface can be seen at both ends of the α7 helices. C, the AphA dimer viewed from the side, colored as in A. The DNA binding surface is at the bottom in this orientation. D, side view of the MarR dimer. The DNA binding and dimerization domains are colored the same as for AphA.
