FIG. 3. Models of protein·DNA complexes.
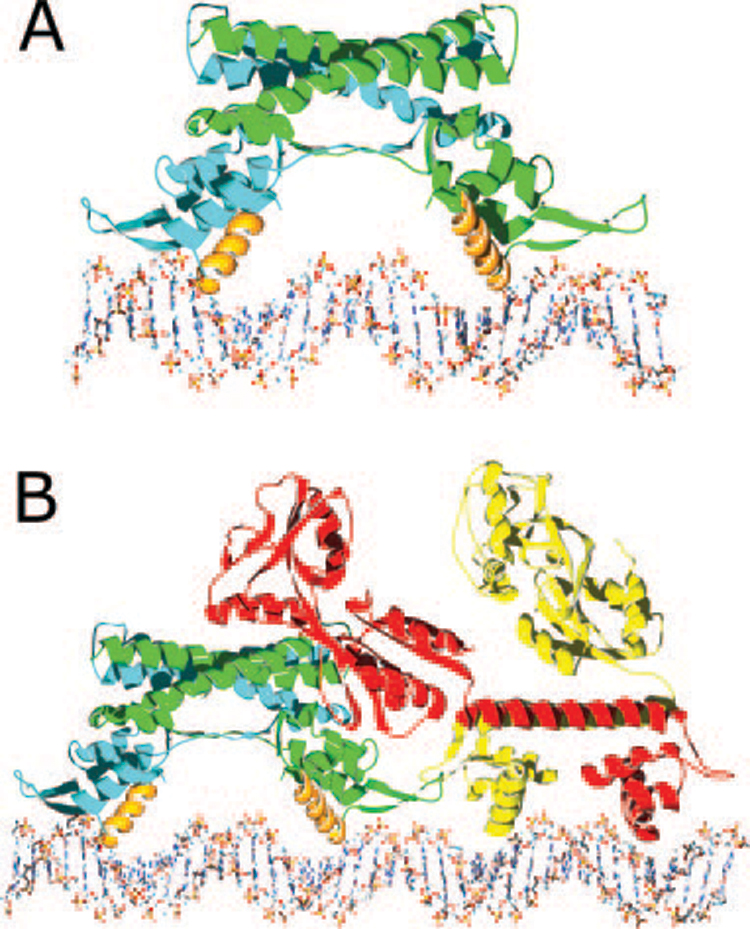
A, a model of the AphA dimer (one chain in blue and the other in green) bound to its DNA site with the complete AphA wing modeled in, based on the wing structure observed in MarR. The primary DNA binding interface is predicted to involve helix α3 from each monomer (orange), which fit into adjacent major grooves on the same face of the DNA. As the helices are not optimally spaced, some conformational change in the protein and/or DNA likely takes place. B, model of the AphA-AphB heterotetramer bound to DNA. The AphB dimer structure was modeled by threading the AphB sequence into the crystal structure of CbnR and then placing it next to the AphA-DNA model shown in A. In this orientation, the C-terminal domain of one molecule of AphB (red) would be able to interact with the AphA dimer (green and blue). Interaction between AphA and AphB could also form between the tips of the wings. This model predicts a linear mode of binding that would not significantly distort the DNA in which four adjacent major grooves are bound by the AphA dimer and the AphB dimer.
