The number of biologically interesting natural products possessing peroxide structure motifs is substantial and still growing.1 Many peroxy natural products display antitumor, anticancer and anti parasite activities, which are attributed to the propensity of the peroxide to initiate radical reactions in an ironrich environment.2 Furthermore, peroxide natural products such as artemisinin are clinically important anti malaria drugs. Despite the potential of chiral peroxides as biologically interesting or even clinically important compounds, synthetic methods for the preparation of chiral peroxides are highly limited.3,4,5,6 In particular efficient catalytic enantioselective peroxidations with simple achiral precursors are urgently needed, yet none are available. In fact only a single example of a chiral auxiliarydirected peroxidation in high diastereoselectivity could be found in the literature.7 Herein, we wish to report the development of a highly enantioselective peroxidation of α,β-unsaturated ketones with an easily accessible chiral organic catalyst.
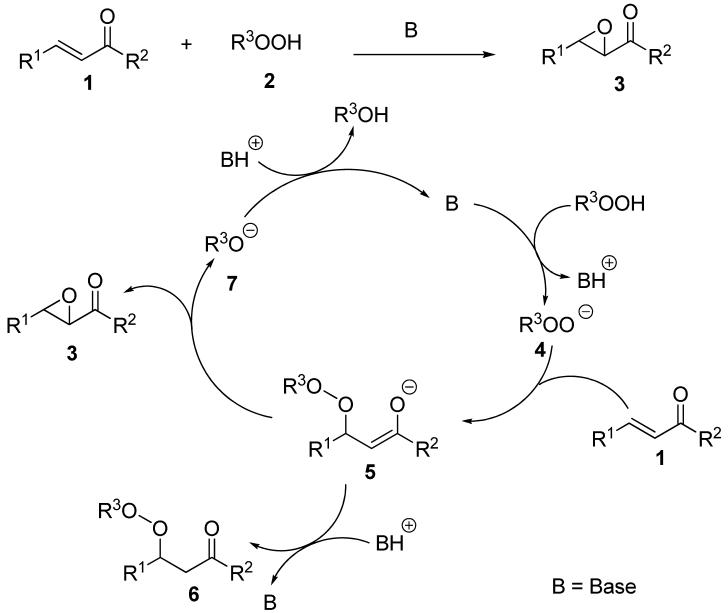
The base-promoted reaction of α,β-unsaturated ketones 1 with hydroperoxides 2 represents a classic epoxidation reaction. Asymmetric variants of this epoxidation with both chiral metal and organic catalysts have also been reported.8-13 It is well-established that the epoxide 3 is formed via a two-step mechanism (Scheme 1); nucleophilic addition of the hydroperoxide 2 to 1 followed by an intramolecular nucleophilic substitution of the resulting enolate (5) that breaks the weak peroxide bond. In principle this epoxidation pathway (1 to 3) could be converted into a peroxidation pathway (1 to 6) if 5 could be trapped by protonation, although the overwhelming preference of 5 for the intramolecular nucleophilic substitution is evident from the lack of reported peroxidation of α,β-unsatrated carbonyl compounds.
Scheme 1.
Mechanism of Base-Catalyzed Nucleophilic Expoxidation of α,β-Unsaturated Ketones
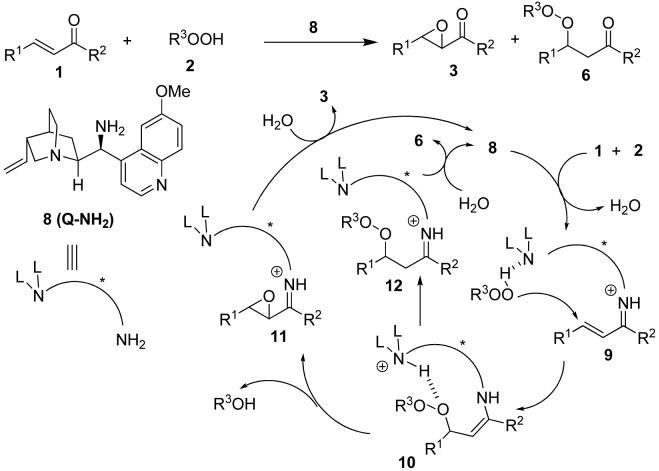
Although chiral amine-catalyzed nucleophilic epoxidations of α,β-unsaturated carbonyl compounds have already been reported,13 we suspected that a cinchona alkaloid derivative such as 814 could not only render the nucleophilic addition of the hydroperoxide 2 to the iminium intermediate 9 enantioselective, but also strongly influence the partitioning of the peroxyenamine intermediate 10 between the epoxidation (10 to 11) and the peroxidation (10 to 12) pathways (Scheme 2). Presumably, due to steric crash and multipoint binding interactions between the peroxyenamine intermediate and the covalently linked cinchona alkaloid, the bond-rotational freedom of the peroxyenamine should be hampered, compared to that of the enolate 5 in Scheme 1. We expected that this conformational rigidity imposed by 8 on the peroxyenamine would diminish its ability to adopt the active conformation by which the nucleophilic enamine moiety is optimally aligned relative to the O-O bond for the nucleophilic attack. This in turn would decelerate the epoxidation. In contrast, with the protonated quinuclidine as a proton source nearby to facilitate the protonation of the peroxyenamine, the peroxidation might be accelerated.
Scheme 2.
A Proposed Catalytic Cycle for the Reaction of 1 and 2 with Cinchona Alkaloid 8
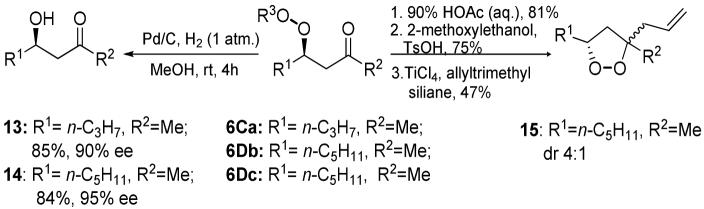
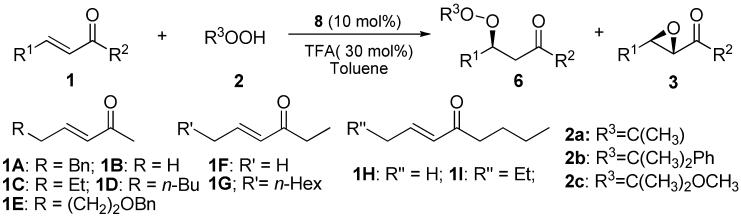
We then investigated how α,β-unsaturated ketone 1A reacted with TBHP (2a) in the presence of 8. We found that, with TFA (20 mol%) as the additive, the reaction afforded the peroxide 6Aa as the dominant product and in 85% ee (entry 1, Table 1). When performed in toluene and with 30 mol% TFA both the peroxide/epoxide (6/3) ratio and the enantioselectivity could be improved to an excellent level (entry 2, Table 1). Importantly, the reaction demonstrated considerable scope for both the α,β-unsaturated ketones 1 and the hydroperoxides 2. Paticularly noteworthy are the highly enantioselective peroxidations of α,β-unsaturated ketones 1 with the α-methoxy isopropyl hydroperoxide 2c (entries 18-23, Table 1). The ability to employ 2c considerably increases the synthetic potential of this new catalytic asymmetric peroxidation, as the corresponding chiral peroxides could be readily converted to chiral hydroperoxides suitable for further elaborations (Scheme 3).15 The catalytic asymmetric peroxidation also provides a new enantioselective route to the chiral β-hydroxy ketones as peroxides could be easily reduced to the corresponding alcohol (Scheme 3).16
Table 1.
Asymmetric Peroxidation of α,β-unsaturated ketones 1 with 8a
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | enone | peroxide | temp(°C) | time(h) | 6/3b | yield(%) 6 | ee (% )c 6 |
| 1d | 1A | 2a | 23 | 2 | 91:9 | nd | 85 |
| 2 | 1A | 2a | 23 | 4 | 92:8 | 85 | 91 |
| 3 | 1B | 2a | 23 | 4 | 94:6 | 88 | 84 |
| 4e | 1C | 2a | 23 | 4 | 94:6 | 91 | 90 |
| 5f | 1D | 2a | 23 | 4 | 93:7(95:5) | 86(90) | 93(90) |
| 6 | 1E | 2a | 23 | 4 | 90:10 | 65 | 91 |
| 7 | 1F | 2a | 23 | 4 | 95:5 | 90 | 89 |
| 8 | 1H | 2a | 23 | 4 | 94:6 | 89 | 87 |
| 9f | 1I | 2a | 23 | 4 | 86:14(93:7) | 64(77) | 94(88) |
| 10 | 1A | 2b | 0 | 16 | 86:14 | 74 | 94 |
| 11 | 1B | 2b | 0 | 12 | 77:23 | 70 | 92 |
| 12 | 1C | 2b | 0 | 12 | 88:12 | 75 | 92 |
| 13 | 1D | 2b | 0 | 16 | 87:13 | 77 | 95 |
| 14 | 1E | 2b | 0 | 12 | 90:10 | 82 | 96 |
| 15 | 1F | 2b | 0 | 24 | 89:11 | 75 | 94 |
| 16 | 1H | 2b | 0 | 24 | 85:15 | 66 | 96 |
| 17 | 1I | 2b | 0 | 24 | 65:35 | 55 | 97 |
| 18g | 1A | 2c | 0 | 19 | 77:23 | 60 | 92 |
| 19g | 1C | 2c | 0 | 24 | 86:14 | 70 | 95 |
| 20g | 1D | 2c | 0 | 24 | 88:12 | 62 | 95 |
| 21g | 1F | 2c | 0 | 17 | 95:5 | 63 | 95 |
| 22g | 1G | 2c | 0 | 24 | 78:22 | 42 | 94 |
| 23g | 1H | 2c | 0 | 24 | 95:5 | 60 | 94 |
Unless noted, reactions were run with 0.3 mmol 1, 0.36 mmol 2, see Supporting Information for details.
Determined by 1H NMR.
see Supporting Information for details.
Reaction was run with 20 mol% TFA in CH2CI2.
Absolute configuration was established as R (see Supporting Inofrmation).
The results in paretheses were obtained with QD-NH2.
Reaction was run with 20 mol% TFA.
Scheme 3.
Synthetic Transformation of Chirall Peroxides 6
Following our observation that the peroxide/epoxide ratio inversely correlated with the reaction temperature, we performed the reactions of various α,β-unsaturated ketones with cumene hydroperoxide (2b) at elevated temperature (23 or 55 °C vs. 0 °C) in order to establish conditions for an asymmetric epoxidation of 1.17 As summarized in Table 2, highly enantiomerically enriched epoxides were indeed obtained as the major product and in synthetically useful yields.18
Table 2.
Asymmetric Epoxidation of α,β-unsaturated ketones 1 with 8a
 | ||||||
|---|---|---|---|---|---|---|
| entry | enone | temp(°C) | time(h) | 6:3b | yield(%) 3 | ee (%)c 3 |
| 1 | 1A | 23 | 72 | 1:99 | 88 | 97 |
| 2 | 1C | 23 | 72 | 1:99 | 91 | 97 |
| 3d | 1D | 23 | 72 | 1:99 | 91 | 97 |
| 4 | 1F | 55 | 24 | 32:68 | 55 | 97 |
| 5 | 1G | 55 | 24 | 33:67 | 54 | 96 |
| 6 | 1H | 55 | 24 | 13:87 | 71 | 97 |
Unless noted, reactions were run with 0.3 mmol of 1, 0.36mmol of 2b, see Supporting Information for details.
Determined by 1H NMR analysis.
Entry 1 was determined by HPLC analysis, others are determined by GC analysis.
Absolute configuration was assigned as (3R, 4S).
In summary, by using a chiral catalyst to not only induce enantioselectivity but also to convert a well established epoxidation pathway into a peroxidation pathway, we have developed the first highly enantioselective catalytic peroxidation reaction. Employing readily available reagents and catalyst, this novel reaction is expected to open new possibility in the asymmetric synthesis of the biologically interesting chiral peroxides. Furthermore, with the same catalyst and reagents, a highly asymmetric epoxidations of acyclic enones could be established simply by performing the reaction at a higher temperature.
Supplementary Material
Acknowledgment
We are grateful for financial support from National Institute of Health (GM-61591). We thank Professor Jeffrey Agar for his assistance in MS analysis.
Footnotes
Supporting Information Available: Experimental procedures and characterization of the products. This material is available free of charge via the internet at http://pubs.acs.org.
References
- (1).For reviews, see: Casteel DA. Nat. Prod. Rep. 1999;16:55. doi: 10.1039/np9920900289.. Rahm F, Hayes PY, Kitching W. Heterocycles. 2004;64:523..
- (2).(a) Posner GH, O'nell PM. Acc. Chem. Res. 2004;37:397. doi: 10.1021/ar020227u. [DOI] [PubMed] [Google Scholar]; (b) Meshnick SR, Thomas A, Ranz A, Xu CM, Pan HZ. Mol. Biochem. Parasitol. 1991;49:181. doi: 10.1016/0166-6851(91)90062-b. [DOI] [PubMed] [Google Scholar]; (c) Posner GH, Oh CH. J. Am. Chem. Soc. 1992;114:8328. [Google Scholar]
- (3).For the preparation of optically active chiral peroxides from optically active oxetanes, see: Dussault PH, Trullinger TK, Noor-e-Ain F. Org. Lett. 2002;4:4591. doi: 10.1021/ol0265259..
- (4).For the preparation of optically active chiral peroxides through the kinetic resolution of racemic chiral peroxides mediated by enantiopure phosphines, see: Driver TG, Harris JR, Woerpel KA. J. Am. Chem. Soc. 2007;129:3836. doi: 10.1021/ja070482f..
- (5).For a peroxidation of allylic and benzylic compounds in modest ee, see: Schulz M, Kluge R, Gelalcha FG. Tetrahedron: Asymmetry. 1998;9:4341..
- (6).For select examples of constructions of chiral peroxide motifs via intramolecular conjugate additions of peroxides to α,β-unsaturated carbonyl compounds, see: O'Neill PM, Searle NL, Raynes KJ, Maggs JL, Ward SA, Storr RC, Park BK, Posner GH. Tetrahedron Lett. 1998;39:6065.. Dussault PH, Eary CT, Woller KR. J. Org. Chem. 1999;64:1789. doi: 10.1021/jo981128q.. Kawanishi M, Kotoku N, Itagaki S, Horii T, Kobayashi M. Bioorg. Med. Chem. 2004;12:5297. doi: 10.1016/j.bmc.2004.04.051..
- (7).Adam W, Güthlein M, Peters E-M, Peters K, Wirth T. J. Am. Chem. Soc. 2001;123:7228. doi: 10.1021/ja010463k. [DOI] [PubMed] [Google Scholar]
- (8).For select reviews of catalytic nucleophilic epoxidation of electrondeficient olefins, see: Porter MJ, Skidmore J. Chem. Commun. 2000:1215.. Nemoto T, Ohshima T, Shibasaki M. J. Synth. Org. Chem. Jpn. 2002;60:94..
- (9).For asymmetric epoxidation of enones with chiral transition metal complexes, see: Bougauchi M, Watanabe S, Arai T, Sasai H, Shibasaki M. J. Am. Chem. Soc. 1997;119:2329.. Nemoto T, Ohshima T, Yamaguchi K, Shibasaki M. J. Am. Chem. Soc. 2001;123:2725. doi: 10.1021/ja004201e..
- (10).For oligopeptide-catalyzed asymmetric epoxidation of enones, see: Julià S, Masana J, Vega JC. Angew. Chem., Int. Ed. Engl. 1980;19:929.. Julià S, Guixer J, Masana J, Rocas J, Colonna S, Annunziata R, Molinari H. J. Chem. Soc., Perkin Trans 1. 1982:1317.. Geller T, Roberts SM. J. Chem. Soc., Perkin Trans. 1. 1999:1397..
- (11).For chiral ketone-based asymmetric epoxidation, see: Wang ZX, Shi Y. J. Org. Chem. 1997;62:8622. doi: 10.1021/jo962392r..
- (12).Phase-transfer catalysis approach, for a review see: Ooi T, Maruoka K. Angew. Chem. Int. Ed. 2007;46:4222. doi: 10.1002/anie.200601737.. For select examples, see: Wynberg H, Marsman B. J. Org. Chem. 1980;45:158.. Lygo B, Wainwright PG. Tetrahedron Lett. 1998;39:1599.. Arai S, Tsuge H, Shioiri T. Tetrahedron Lett. 1998;39:7563.. Corey EJ, Zhang F-Y. Org. Lett. 1999;1:1287. doi: 10.1021/ol990964z.. Ooi T, Ohara D, Tamura M, Maruoka K. J. Am. Chem. Soc. 2004;126:6844. doi: 10.1021/ja048600b..
- (13).For chiral amine-catalyzed nucleophilic asymmetric epoxidations of carbonyls, see: Marigo M, Franzén J, Poulsen TB, Zhuang W, Jørgensen KA. J. Am. Chem. Soc. 2005;127:6964. doi: 10.1021/ja051808s.. Lattanzi A. Org. Lett. 2005;7:2579. doi: 10.1021/ol050694m.. Lee S, MacMillan DWC. Tetrahedron. 2006;62:11413.. Li Y, Liu X, Yang Y, Zhao G. J. Org. Chem. 2007;72:288. doi: 10.1021/jo0617619.. Wang X, List B. Angew. Chem. Int. Ed. 2008;47:1119. doi: 10.1002/anie.200704185..
- (14).For reports of 8-catalyzed enantioselective reactions, see: Xie J-W, Chen W, Li R, Zeng M, Du W, Yue L, Chen Y-C, Wu Y, Zhu J, Deng J-G. Angew. Chem. Int. Ed. 2007;46:389. doi: 10.1002/anie.200603612.. Xie J-W, Yue L, Chen W, Du W, Zhu J, Deng J-G, Chen Y-C. Org. Lett. 2007;9:413. doi: 10.1021/ol062718a.. Bartoli G, Bosco M, Carlone A, Pesciaioli F, Sambri L, Melchiorre P. Org. Lett. 2007;9:1403. doi: 10.1021/ol070309o.. McCooey SH, Connon SJ. Org. Lett. 2007;9:599. doi: 10.1021/ol0628006.. Singh RP, Bartelson K, Wang Y, Su H, Lu X, Deng L. J. Am. Chem. Soc. 2008;130:2422. doi: 10.1021/ja078251w..
- (15).(a) Dussault PH, Sahli AS. J. Org. Chem. 1992;57:1009. [Google Scholar]; (b) Ahmed A, Dussault PH. Tetrahedron. 2005;61:4657. [Google Scholar]; (c) Dussault PH, Liu X. Org. Lett. 1999;1:1391. doi: 10.1021/ol990954y. [DOI] [PubMed] [Google Scholar]; (d) Dai P, Dussault PH. Org. Lett. 2005;7:4333. doi: 10.1021/ol051407h. [DOI] [PubMed] [Google Scholar]; (e) Xu C, Raible JM, Dussault PH. Org. Lett. 2005;7:2509. doi: 10.1021/ol050291m. [DOI] [PubMed] [Google Scholar]; (f) Dai P, Trullinger TK, Liu X, Dussault PH. J. Org. Chem. 2006;71:2283. doi: 10.1021/jo0522254. [DOI] [PubMed] [Google Scholar]
- (16).For asymmetric approaches for the preparations of β-hydroxy carbonyls via enantioselective catalytic conjugate additions, see: Vanderwal CD, Jacobsen EN. J. Am. Chem. Soc. 2004;126:14724. doi: 10.1021/ja045563f.. Bertelsen S, Diner P, Johansen RL, Jørgensen KA. J. Am. Chem. Soc. 2007;129:1536. doi: 10.1021/ja068908y.. Carlone A, Bartoli G, Bosco M, Pesciaioli F, Ricci P, Sambri L, Melchiorre P. Eur. J. Org. Chem. 2007:5492. doi: 10.1021/ol070309o..
- (17).For an aspartate-catalyzed asymmetric electrophilic olefin epoxidation, see: Peris G, Jakobsche CE, Miller SJ. J. Am. Chem. Soc. 2007;129:8710. doi: 10.1021/ja073055a..
- (18).While this manuscript was in preparation, an 8-catalyzed asymmetric epoxidation of cyclic enones with H2O2 was reported: Wang X, Reisinger CM, List B. J. Am. Chem. Soc. 2008;130:6070. doi: 10.1021/ja801181u..
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.