Table 1.
Asymmetric Peroxidation of α,β-unsaturated ketones 1 with 8a
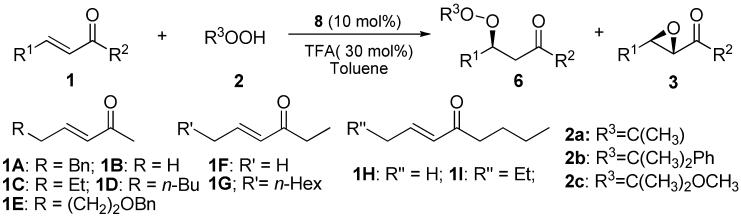 | |||||||
|---|---|---|---|---|---|---|---|
| entry | enone | peroxide | temp(°C) | time(h) | 6/3b | yield(%) 6 | ee (% )c 6 |
| 1d | 1A | 2a | 23 | 2 | 91:9 | nd | 85 |
| 2 | 1A | 2a | 23 | 4 | 92:8 | 85 | 91 |
| 3 | 1B | 2a | 23 | 4 | 94:6 | 88 | 84 |
| 4e | 1C | 2a | 23 | 4 | 94:6 | 91 | 90 |
| 5f | 1D | 2a | 23 | 4 | 93:7(95:5) | 86(90) | 93(90) |
| 6 | 1E | 2a | 23 | 4 | 90:10 | 65 | 91 |
| 7 | 1F | 2a | 23 | 4 | 95:5 | 90 | 89 |
| 8 | 1H | 2a | 23 | 4 | 94:6 | 89 | 87 |
| 9f | 1I | 2a | 23 | 4 | 86:14(93:7) | 64(77) | 94(88) |
| 10 | 1A | 2b | 0 | 16 | 86:14 | 74 | 94 |
| 11 | 1B | 2b | 0 | 12 | 77:23 | 70 | 92 |
| 12 | 1C | 2b | 0 | 12 | 88:12 | 75 | 92 |
| 13 | 1D | 2b | 0 | 16 | 87:13 | 77 | 95 |
| 14 | 1E | 2b | 0 | 12 | 90:10 | 82 | 96 |
| 15 | 1F | 2b | 0 | 24 | 89:11 | 75 | 94 |
| 16 | 1H | 2b | 0 | 24 | 85:15 | 66 | 96 |
| 17 | 1I | 2b | 0 | 24 | 65:35 | 55 | 97 |
| 18g | 1A | 2c | 0 | 19 | 77:23 | 60 | 92 |
| 19g | 1C | 2c | 0 | 24 | 86:14 | 70 | 95 |
| 20g | 1D | 2c | 0 | 24 | 88:12 | 62 | 95 |
| 21g | 1F | 2c | 0 | 17 | 95:5 | 63 | 95 |
| 22g | 1G | 2c | 0 | 24 | 78:22 | 42 | 94 |
| 23g | 1H | 2c | 0 | 24 | 95:5 | 60 | 94 |
Unless noted, reactions were run with 0.3 mmol 1, 0.36 mmol 2, see Supporting Information for details.
Determined by 1H NMR.
see Supporting Information for details.
Reaction was run with 20 mol% TFA in CH2CI2.
Absolute configuration was established as R (see Supporting Inofrmation).
The results in paretheses were obtained with QD-NH2.
Reaction was run with 20 mol% TFA.
