Abstract
Red tides in the Gulf of Mexico are formed by blooms of the dinoflagellate Karenia brevis, which produces brevetoxins (PbTx). Brevetoxins can be transferred from water to air in the wind-powered whitecapped waves during red tide episodes. Inhalation exposure to marine aerosol containing PbTx causes respiratory problems. A liquid chromatograph/ tandem mass spectrometric method was developed for the detection and quantitation of several PbTxs in ambient samples collected during red tide events. This method was complemented by a previously developed antibody assay that analyzes the entire class of PbTx compounds. The method showed good linearity, accuracy, and reproducibility, allowing quantitation of PbTx compounds in the 10 pg/m3 range. Air concentrations of PbTxs and brevenal for individual samples ranged from 0.01 to 80 ng/m3. The particle size showed a single mode with a mass median diameter between 6 and 10 μm, which was consistent for all of the PbTx species that were measured. Our results imply that individual PbTxs were from the same marine aerosol or from marine aerosol that was produced from the same process. The particle size indicated the likelihood of high deposition efficiency in the respiratory tract with the majority of aerosol deposited in the upper airways and small but not insignificant deposition in the lower airways.
Introduction
Red tides in the Gulf of Mexico are commonly formed by the fish-killing dinoflagellate Karenia brevis (formerly known as Gymnodinium breve) (1). The organism produces as many as nine potent polyether brevetoxins, called PbTxs, designated PbTx-1, PbTx-2, etc., that result in the death of a massive number of fish (2), mammals (3), and other marine species during red tide blooms. PbTxs can also concentrate in the tissues of shellfish when they feed on dinoflagellates (4). People who eat these shellfish may suffer from neurotoxic shellfish poisoning, a food poisoning that can cause severe gastrointestinal and neurological symptoms.
Brevetoxins can also be transferred from water to air in the wind-powered whitecap waves during the red tide episodes (5). Inhalation exposure to marine aerosol containing brevetoxins causes respiratory problems including involuntary coughing and sneezing, watery eyes, rhinorrhea, a burning sensation in the throat and nose, and difficulty breathing (6–8). Low air concentrations of brevetoxins, in the range of 3–4 ng/m3, could initiate problems in the upper respiratory tract, including cough, irritation in the throat, and itchy eyes (8).
Several techniques have been used to detect and quantify brevetoxins. Biochemical immunoassay techniques including radioimmunoassay (9) and enzyme-linked immunosorbent assays (ELISA) (10, 11) have high sensitivity and usually can detect a class of compounds that react with a specially prepared antibody. The ELISA technique does not give information on specific PbTx species. Studies have shown that the relative toxicity of the PbTx compounds is not all the same (12, 13). During the stationary and declining phases of red tides, the toxin profile in cells changes markedly. The concentrations of PbTx-1 and PbTx-2 decrease substantially, and PbTx-3 increases (14). There are also indications that the PbTx profile changes with different clones of K. brevis (15).
This paper reports concentration and size distribution of both total (ELISA) and speciated PbTx species collected on beaches during red tide algal blooms. A liquid chromatographic/tandem mass spectrometric (LC/MS/MS) method has been developed and evaluated for determining individual PbTx species in air sample extracts. The assay has been developed in response to a need to determine these compounds at low concentrations and in complex matrixes. Previous LC techniques for speciating PbTx species have been developed (Pierce (7, 16) with an ultraviolet detector and Hua (17) for a single MS), but their detection limits are not low enough to determine pg/m3 quantities of some of the PbTx compounds in sampling periods from 4 to 8 h. Even at higher aerosol red tide concentrations, this technique may only detect and quantify major components such as PbTx-2 and -3 (7) and miss other PbTxs with concentrations below the limit of detection (LOD). In addition, ultraviolet detection is not selective enough to ensure that there are no interferences from other compounds during the analysis. The MS/ MS assay reported in this paper offers improved sensitivity compared with the previously reported MS method along with improved selectivity in the air sample matrix by monitoring both the molecular ion of the PbTx compound in the first MS and its collision-induced fragment ion in the second MS.
PbTx concentrations as well as particle size distributions of red tide aerosol samples during several red tide blooms in Florida are analyzed using both LC/MS/MS and ELISA techniques. This information is used to estimate inhaled doses of red tide aerosol. It also has implications in understanding the aerosol production sources.
Experimental Section
Sample Collection
Field studies were performed in Sarasota, FL, from 2001 to 2003. Air samplers were set up along Siesta Beach and Lido Beach to collect marine aerosols. High-volume air samplers (Model G2000H, Andersen Instruments, Smyrna, GA) were placed near the water to collect large quantities of material for analysis of PbTx. Some samplers collected airborne particles in one filter substrate for total suspended aerosol concentrations, whereas other samplers housed a five-stage, high-volume cascade impactor (Model SA235, Andersen Instruments) for total concentration as well as particle size distribution. Glass-fiber filters (20 cm × 25 cm) were used as the collection substrates (Whatman EPM2000, Whatman International Ltd., Maidstone, UK) for the single-stage filter sampler. Cellulous filters (15 cm × 14 cm) (Filter Paper 41, Whatman International Ltd.) were used for the five-stage cascade impactor. The sampling time usually started between 8:00 a.m. and 8:30 a.m. and ended between 3:30 p.m. and 4:30 p.m. During these time periods, human volunteers including life guards and sensitive population were also at the beaches (6, 8). After collection, samples were stored at ambient temperature for 1–2 days until shipment to Lovelace by Federal Express Overnight. After receipt at Lovelace, filters were stored at −20 °C until further processing.
Seawater samples were collected in 1-L glass bottles three times each day (8:30 a.m., noon, and 4:00 p.m.) from the surf zone adjacent to each air sampler location to provide an indication of changes in cell counts and toxin composition throughout each day. A 20-mL subsample was collected from each bottle and fixed with Utermohls solution for microscopic identification and enumeration of K. brevis cells. The remaining water sample was processed for brevetoxin analysis by LC/MS according to the procedure of Pierce et al. (7) and for verification by ELISA according to the procedure of Naar et al. (11).
Sample Extraction and Preparation
During method development both sonication and Soxhlet extraction were evaluated as potential approaches for extraction of the filter samples. To evaluate each extraction technique, PbTx-2 was spiked directly onto the filters at a concentration of ∼100 and 1000 ng and allowed to dry at room temperature for approximately 60 min. For Soxhlet extraction, acetone, ethyl acetate, dichloromethane, and methanol (all solvents analytical grade from Fisher Scientific) were all evaluated by placing the filters directly into the Soxhlet and extracting for 24 h. After extraction, the solvent was evaporated under nitrogen (as described below) and analyzed by LC/MS/MS. The Soxhlet extraction was compared with the same solvents extracted by sonication and liquid–liquid extraction as follows: filters were cut in half with laboratory scissors and then one-half of the filter was folded over (exposed side in) and rolled into a 15-mL polypropylene tube (second half of filter was stored at −20 °C). Then 10 mL of analytical grade acetone (Fisher Scientific) was added to the tube and the sample was vortexed for 20 s, sonicated for 2 min, and then placed on a circular rotator (Roto-Torquie, low speed #10, Cole-Parmer Instruments, Vernon Hills, IL) for 20 min. After rotation, the filter was removed from the extraction tube and the 10-mL extract was then evaporated under a gentle stream of nitrogen to ∼100 μL, vortexed for 5 s to reconstitute the extract, and recombined with 50:50 methanol:purified water (PW) to the final analysis volume (200 μL). The final volume of the extract was determined by visible analysis of the meniscus compared with a sample vial that contained a measured volume of solvent. Each of the extraction techniques and solvents were evaluated for extraction efficiency by comparing the absolute concentration in the filter extracts to PbTx-2 spiked directly into solvents without extraction.
Among the techniques and solvents, acetone coupled to sonication and rotation showed the best recovery (>50% for PbTx-2). All other solvents and the Soxhlet approach showed extraction efficiencies less than 30–40%. Because some compounds will “stick” to polypropylene tubes, the use of glass and silanized glass tubes for the extraction was also evaluated, and their use did not increase the extraction efficiency. On this basis, the acetone/sonication/rotation method was used as the final method for sample extractions, and the recovery analysis was extended to the remainder of the PbTx species. For the final analysis, stored filters were removed from −20 °C storage and equilibrated to room temperature prior to extraction as described above.
Calibration Standards
Neat PbTx species were obtained from our colleague at the University of North Carolina at Wilmington. The compounds were synthesized/purified as previously described (15). Calibration stock standards were preweighed gravimetrically and constituted in acetone. To account for extraction efficiency, with every lot of samples, calibration standards were made by spiking stock solutions directly on to the same type of filters that were extracted for PbTx determination. For each calibration level, three filters were spiked in triplicate with 100 μL of a stock solution that was diluted to a concentration to achieve the targeted mass on the filter. The filters were then air-dried in the laboratory for 30 min and then processed through the sample extraction process described below. Calibration curves were created by averaging the three filters at each calibration level. Five calibration solutions spanned from the limit of quantitation (LOQ) to ∼500 ng/mL.
Instrumental Conditions and Data Analysis
LC separation was conducted with a Shimadzu LC-10 ADVP (Shimadzu, Kyoto, Japan) fitted with a C18 “Aqua”column [3 μm, 125 A, 75 × 200 mm, (Phenomenex #003–4311-B0, Torrance, CA)] and C18 guard column (Phenomenex #AJO4287, Torrance, CA). The column temperature was set at 32 °C (Eppendorf TC-50, Fisher Scientific). The flow rate was 150–200 μL/min. The injection volume was 50 μL with a 100-μL overfill (autoinjector). The solvents used for LC separation were PW (solvent A) and methanol:1 mM ammonium acetate (solvent B) that were operated on the following time cycle: 20% B for 0–1 min, linear gradient to 90% B at 3 min, 90% B 3–10 min, 20% B for 10.1–12.5 min.
MS/MS was performed using an Applied Biosystems (Foster City, CA) 365 instrument managed by the Analyst Version 1.3.1data station. Analytes were ionized using a heated nebulizer ionization source. Ionization and MS conditions are summarized in Table 1. The instrument was operated in the multiple reaction monitor mode, which allows analysis of both the molecular ion (typically unaltered parent compound) and the fragment ion (daughter ion) that is produced by collision-induced disassociation. Specific PbTx species were quantified by ratios of the parent/daughter ion pairs of m/z 867.5/849.5 (PbTx-1), 895.5/877.5 (PbTx-2), 897.5/ 725.4 (PbTx-3), 911.6/ 893.7 (PbTx-6), 899.6/863.9 (PbTx-9), and 657.4/273.2 (brevenal).
TABLE 1. Source and MS Operational Settings for the LC/MS/MS PbTx Method.
| Source Parameters | |
| nebulizer gas setting | 8 |
| curtain gas setting | 10 |
| ion spray voltage (V) | 4800 |
| temperature (°C) | 400 |
| MS Parameters | |
| declustering potential (V) | 36 |
| focusing potential (V) | 204 |
| collision energy (V) | 30 |
| collision cell exit potential (V) | 30 |
LC/MS/MS Assay Performance Qualification
Assay accuracy was assessed via duplicate spike experiments with 75 ng of each PbTx. Spikes were made directly onto filters and extracted. Assay accuracy was defined as the percent deviation from the spiked concentrations. Instrument precision and extraction precision were assessed on the basis of three simultaneous extractions at each calibration level. Precision was calculated on the basis of the coefficient of variation (CV), determined by the (standard deviation/average) ×100. Compound specificity was assessed by verifying no interference in the extraction matrix. The LOQ was defined as the lowest concentration that can be injected six times and achieve precision with a CV of less than 20%. The limit of detection (LOD) was set at the lowest standard that yields 3 times the baseline signal of PbTx present in blank samples. The linear range is defined from the LOQ to the highest standard (500 ng) used in the assay with linearity. The acceptance criterion for linearity is a coefficient of determination (R2) of 0.95 over the working range.
Quality control measures for the LC/MS/MS assay included analysis of unexposed filters as “blanks” to assess the potential for background contamination from the filters to contribute to the observed instrument responses. No subtractions of blank values were made because the blanks were all void of any PbTx or PbTx interferences. Analytical precision was assessed for each batch of samples by triplicate extraction of each calibration/filter spike and by duplicate analysis of at least 10% of the samples (selected randomly). Measurement precision was calculated for each PbTx species and then utilized along with the LOQ to define the confidence interval (analytical uncertainty) according to the following equation
| (1) |
where a = the replicate precision, b = the analyte concentration, and c = the analyte detection limit. Concentration data with uncertainty values higher or equal to their concentrations had a low degree of measurement confidence and were considered below quantifiable limits if detected.
ELISA and Sodium Analyses
Each impactor substrate (two 1.5 cm2 portions of filter on each impactor stage, obtained with laboratory scissors) was analyzed by a competitive ELISA described elsewhere (11), based on the specific activity of the goat anti-PbTx antibody (10). The analysis provided a total amount of PbTxs but not the individual PbTxs. The LOQ of the brevetoxins using the ELISA assay was 0.6 ng/sample.
About 90-cm2 sections (cut with laboratory scissors) of cellulose filter substrates from the high-volume impactor were extracted with 25 mL of ionic strength adjustor (ISA) solution and then its sodium content was determined by sodium ion selective electrodes (Fisher Scientific) (18). The standard curve of this method was established by spiking known amounts of NaCl (to mimic natural NaCl in seawater that may impact response of MS) in the cellulose filter and then extracting it with the same procedure. The standard curve was linear in the range of 1–1000 μg/mL of sodium chloride. Cellulose filters have low sodium background and therefore the detection limit of this method was 1 μg.
Particle Size Analysis
The high-volume impactor sampler was operated at a flow rate of about 600 L/min. The cutoff diameters for the impactor stages were 10, 6.2, 3, 1.9, 0.9, and 0.25 μm, respectively. The particle size analysis was processed by using SigmaPlot software (SPSA, Chicago, IL). The particle size data was fitted with a log-normal distribution. The mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD) for each distribution were obtained by using the nonlinear least-squares fit in the SigmaPlot software.
Dosimetery Modeling
The deposition pattern of the inhaled red tide aerosol in different regions of the respiratory tract can be estimated using the ICRP 66 lung model (19). The human respiratory tract is divided into three anatomical regions: The extrathoracic (ET) airway, including the naso-oro-pharyngo-laryngeal (NOPL) region, is the entry to the respiratory tract and the first defense against hazardous inhaled material. The tracheobronchial (TB) tree region includes the trachea and 16 generations of branching airways. Gas exchange takes place in the pulmonary region (P). Particles deposit in the lung by inertial impaction, sedimentation, diffusion, and electrostatic mechanisms.
Assuming a breathing rate of 25 L/min with light exercise for an adult male and measured particle size distribution, we calculated the deposition fractions of inhaled particles in the three regions of the human respiratory tract using LUDEP software (NRPB, Oxon, UK), which is based on the ICRP lung model (19).
Results and Discussion
Evaluation of the LC/MS/MS Method
Table 2 summarizes the performance of the extraction and LC/MS/MS assay for PbTx-1, -2, -3, -6, -9, and brevenal. All compounds satisfied the predefined criteria for assay performance (linearity, precision, etc). The LOQ for the compounds was ∼0.4 ng and the LOD was ∼0.04 ng. Although the extraction efficiency was less than desirable, especially for PbTx-1 and brevenal, the precision of the extraction was good, and the extraction efficiency for each analyte was linear across the range of the assay. Because the extraction efficiency did not change with changes in concentration, it was considered acceptable to conduct the analysis with lower (than desired) extraction efficiency.
TABLE 2. LC/MS/MS Method Performance Characteristics for PbTx Analysis.
| linerarity, R2 | accuracy (% deviation) |
% recovery | precision, CVa (%) | LOQb (ng/sample) |
|
|---|---|---|---|---|---|
| PbTx1 | 0.99 | 13 | 27 | 11 | 0.6 |
| PbTx2 | 0.99 | 5 | 54 | 6 | 0.4 |
| PbTx3 | 0.99 | 9 | 66 | 9 | 0.4 |
| PbTx6 | 0.99 | 15 | 59 | 7 | 0.4 |
| PbTx9 | 0.99 | 7 | 61 | 10 | 0.4 |
| brevenal | 0.99 | 12 | 35 | 12 | 0.4 |
Coefficient of variation.
Limit of quantitation.
The analyses were performed at the concentration range of 0.8–500 ng/mL.
As indicated previously, the extraction efficiency was determined by spikes of PbTx compounds onto filters prior to extraction. This approach has limitations, as it is uncertain if PbTx spiked directly onto a filter will bind to the filter the same way as PbTxs that were sampled from the air (and are accompanied by other materials from ambient air).
Assay selectivity was determined by assaying blank filter/nonbeach samples to show zero response for the PbTx species (no PbTx were observed). By monitoring both the molecular ion and the daughter ion of each compound by MS/MS, the instrument selectively “filters” out most other materials that are in the complex air matrix (enhancing selectivity/accuracy of the assay). An example of a “clean” chromatogram from an ambient sample is shown in Figure 1, where the PbTx species are clearly distinguished from the baseline of an ambient sample. This spectra showed PbTx-1, -2, -3–6, and -9 with higher concentrations for PbTx-2 and -3. Brevenal was not observed in this sample.
FIGURE 1.
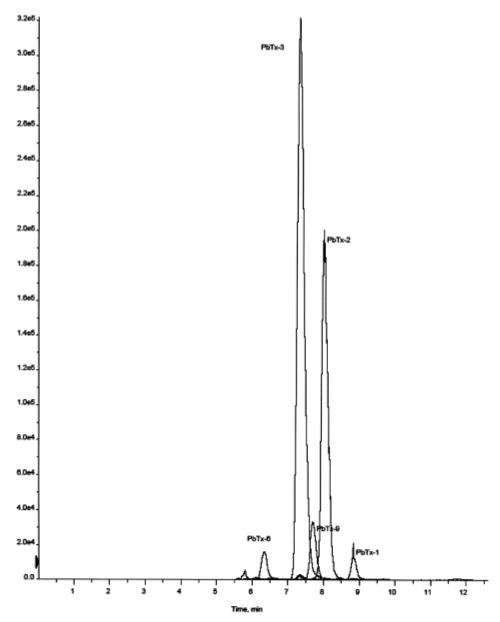
Chromatogram of a red tide aerosol sample.
The stability of PbTx under long term storage and other conditions has yet to be thoroughly evaluated. The stability of extracts analyzed in the time frame of the current study is believed to be good, because reanalysis within days shows good agreement. Future studies will evaluate the long-term stability of PbTx.
Analysis of Air Samples
The brevetoxin profiles in water and aerosol samples from a March 2003 study at Siesta Beach are shown in Figure 2. The data showed that in both water and air samples, PbTx-2 and -3 were the major PbTx species. Much lower concentrations of PbTx-1 and -9 were also observed. Trace amounts of brevenal were detected in both water and air samples. This was the first report of brevenal, an antagonist of toxic effects of brevetoxins previously discovered in the K. brevis culture (20), being detected in the environment. Air concentrations of PbTxs for individual samples ranged from 0.01 to 80 ng/m3.
FIGURE 2.
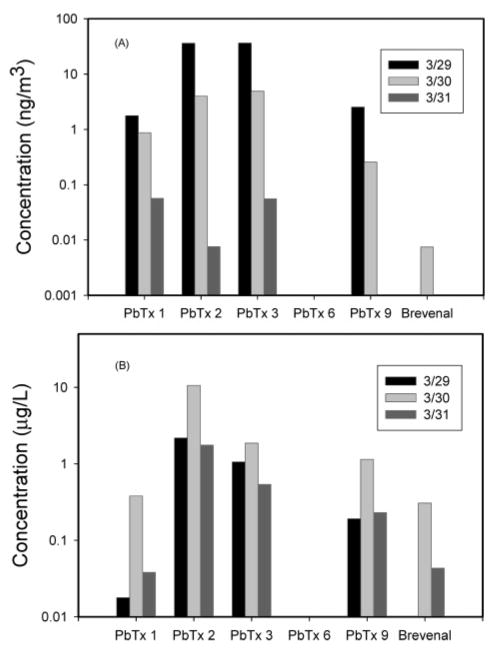
PbTx profiles for air (A) and water (B) samples obtained at Siesta Beach in March 2003.
Comparison of LC/MS/MS and ELISA Analysis
For each sample, both ELISA and LC/MS/MS analyses provided a measure of PbTx concentrations. Figure 3A shows the comparison of total aerosol concentrations (from summation of concentrations on all stages of impactor samples or from filter samples) from these two analyses. These data included all aerosol samples taken in September 2001 and May 2002 at Lido and Siesta Beach as well as samples taken in 2003 at Siesta Beach. In general, the concentrations based on ELISA analysis are higher than those of LC/MS analysis. At low concentrations (<50 ng/m3 for ELISA concentration), the relationship appeared to be linear with the ratio of LC/MS/ MS to ELISA concentrations close to 0.5. However, as the concentration increased, this linear relationship did not hold. A best fitted equation using a nonlinear least-squares regression (SigmaPlot, SPSS, Chicago, IL) is
FIGURE 3.
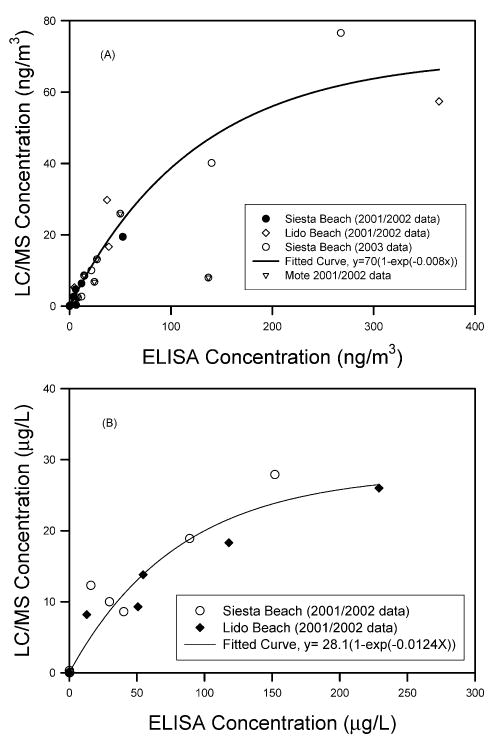
Comparison of brevetoxin concentrations as determined from ELISA and LC/MS analyses of air samples (A) and water samples (B).
| (2) |
Figure 3B shows the comparison of brevetoxin concentrations in water samples taken in the same field studies. Similarly, the ELISA analysis provided a higher concentration than the LC/MS/MS analysis. A best fitted equation using a nonlinear least-squares regression (SigmaPlot) is
| (3) |
The LC/MS/MS is more specific and provided information for each brevetoxin and related species. However, it requires standards for the known brevetoxins for analysis and may miss other compounds that have not been identified. The ELISA technique has high sensitivity but is not specific to individual brevetoxins. It detects a class of compounds that are reactive to the specific antibody. ELISA requires a small number of samples and does not need elaborated extraction for the analysis. Our results showed that in both air and water samples the brevetoxin concentrations from ELISA analysis were higher than those of LC/MS/MS analysis. This was reasonable because for LC/MS/MS analysis we only detected and quantified brevenal, PbTx-1, -2, -3, -6, and -9, whereas the ELISA analysis detected all compounds that have a chemical structure similar to the binding site of the ELISA. Though the ELISA is likely measuring additional compounds that the LC/MS/MS is not measuring, the specificity of the ELISA for analogous compounds has not been confirmed. Certainly there are other components of red tide aerosol that remain to be identified and analyzed.
Particle Size Distribution
Figure 4A shows an example of a particle size distribution obtained during a red tide episode with a high PbTx concentration of 77 ng/m3 obtained at Siesta Beach in the March 2003 study. The particle size distribution for PbTx-1, -2, -3, and -9 were similar, indicating that individual PbTxs were produced from the same marine aerosol. Similarly, Figure 4B shows the particle size distribution for a low PbTx concentration of 1.6 ng/m3 obtained at Siesta Beach in the May 2002 study. The particle size distribution of PbTx-2, -3, and -9 were also similar. The size distribution showed a range of particles from 0.5 to 20 μm with a single size mode of approximately 6–12 μm. As shown in Figure 4, the particle size can be described with a log-normal size distribution:
FIGURE 4.
Normalized red tide aerosol size distribution as determined from an impactor sample obtained at Siesta Beach on 3/29/2003 (A) and 5/3/2002 (B). The histograms are data for individual PbTx components. The fitted curve is for the total PbTx concentration with a MMAD of 6.4 μm and a GSD of 1.67 (A), and a MMAD of 6.9 μm and a GSD of 1.83 (B).
| (4) |
where xo is the MMAD and σg is the GSD. The best-fit curve using SigmaPlot software as shown in parts A and B of Figure 4 has a MMAD of 6.4 and 6.9 μm and a GSD of 1.67 and 1.83, respectively. The MMAD and GSD of the fitted log-normal distribution of red tide aerosol from several field studies are listed in Table 3. This particle size information was then used to estimate the dose of inhaled red tide aerosol in the human volunteers as shown in the following section.
TABLE 3. Summary of Red Tide Aerosol Size Distribution (Mean and SD).
| MMAD (μm) | GSD | |
|---|---|---|
| 10/00, Corpus Christia | 8.04 ± 1.03 | 1.60 ± 0.03 |
| 9/01, Siesta Beach | 9.55 ± 2.30 | 1.80 ± 0.08 |
| 9/01, Lido Beach | 9.25 ± 1.48 | 1.76 ± 0.20 |
| 5/02, Siesta Beach | 6.40 ± 0.74 | 1.87 ± 0.06 |
| 1/03, Siesta Beach | 5.99 ± 1.37 | 1.91 ± 0.02 |
| 3/03, Siesta Beach | 6.54 ± 1.34 | 1.73 ± 0.05 |
Data published in ref 8.
In addition to LC/MS/MS and ELISA analyses of impactor samples, we also analyzed the sample for sodium chloride because it is the major component of marine aerosols. Figure 5 shows particle size distributions for these three analyses from samples taken on 3/29/2003 in Siesta Beach. Figure 5A shows that distribution of NaCl has a coarse-size mode with diameter in the range of 4–20 μm and a fine-size mode with diameter of 0.1–0.6 μm. The sodium chloride concentration was 62 μg/m3, in which the coarse-mode particles had 72% of the mass. The sodium chloride concentration was in the range of observed sea salt aerosol concentration (21). Figure 5B shows the comparison of three analyses from the same impactor samples. The PbTx concentrations for the ELISA and LC/MS/MS analyses were 140 and 40 ng/m3, respectively. The PbTxs were mainly in the coarse-mode of the marine aerosol. There was very little PbTxs in the fine particle mode less than 1 μm.
FIGURE 5.
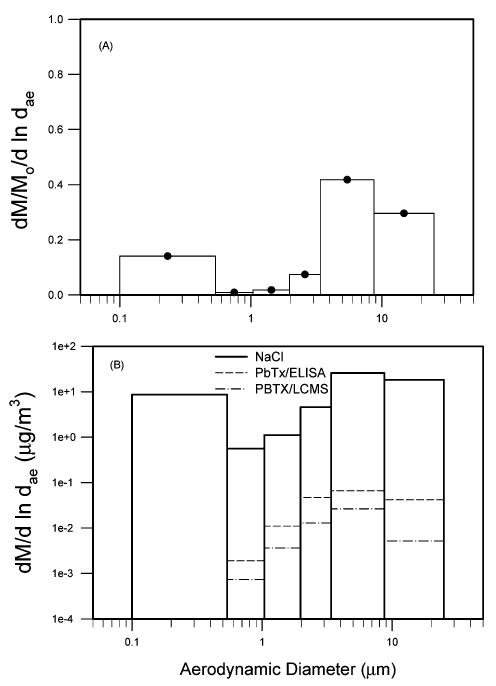
Normalized red tide aerosol size distribution for sodium chloride (A) as determined from an impactor sample obtained at Siesta Beach on 5/3/2002. Panel B compares the absolute size distributions for sodium analysis, ELISA, and LC/MS/MS.
The particle size distribution of brevetoxin showed that the red tide aerosol consisted of coarse particles with MMAD in the range of 6–12 μm, similar to what we observed in previous studies (8). The red tide aerosol may be produced by a breakup of bubbles from whitecap waves (5). Measured size distributions of marine aerosols showed a bimodal distribution with a peak in the fine-particle mode (0.1–0.2 μm) and another peak in the coarse-particle mode (2–30 μm) (21). Our sodium analysis was consistent with the literature. The red tide aerosol appears to be a component of marine coarse particles associated with sea salts.
Dosimetry of Inhaled PbTx
The fractional deposition of inhaled red tide aerosol in the human respiratory tract was estimated on the basis of the size measurements as listed in Table 4. A total deposition fraction of 76–90% was calculated with the majority of aerosol deposited in the ET region or upper airway (75–84%) and small but not insignificant deposition (2–6%) in the lower airways (TB and P). The inhaled red tide aerosol has high deposition efficiency in the respiratory tract and the pattern of deposition would help to explain the observed upper airway irritation (i.e., eye irritation, nasal congestion, and cough) (22). The dose rate of the deposited brevetoxins can be calculated from Table 3 for a breathing rate of 25 L/min and for a unit air concentration of 1 ng/m3 of PbTx as shown in Table 5.
TABLE 4. Estimated Deposition Fractions in the Human Respiratory Tract.
| extrathoracic | TB | pulmonary | total | |
|---|---|---|---|---|
| 10/00, Corpus Christi | 0.83 ± 0.02 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.86 ± 0.03 |
| 9/01, Siesta Beach | 0.79 ± 0.04 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.82 ± 0.05 |
| 9/01, Lido Beach | 0.80 ± 0.03 | 0.02 ± 0.00 | 0.01 ± 0.01 | 0.83 ± 0.04 |
| 5/02, Siesta Beach | 0.82 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.88 ± 0.01 |
| 1/03, Siesta Beach | 0.83 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.88 ± 0.03 |
| 3/03, Siesta Beach | 0.82 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.01 | 0.89 ± 0.01 |
TABLE 5. Estimated Dose Rate of PbTx (ng/h) in the Human Respiratory Tract for a PbTx Concentration of 1 ng/m3.
| extrathoracic | TB | pulmonary | total | |
|---|---|---|---|---|
| 10/00, Corpus Christi | 1.25 ± 0.03 | 0.03 ± 0.01 | 0.02 ± 0.01 | 1.30 ± 0.04 |
| 9/01, Siesta Beach | 1.18 ± 0.05 | 0.03 ± 0.01 | 0.02 ± 0.01 | 1.23 ± 0.08 |
| 9/01, Lido Beach | 1.19 ± 0.05 | 0.03 ± 0.01 | 0.02 ± 0.01 | 1.24 ± 0.05 |
| 5/02, Siesta Beach | 1.23 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.01 | 1.31 ± 0.01 |
| 1/03, Siesta Beach | 1.25 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.02 | 1.33 ± 0.04 |
| 3/03, Siesta Beach | 1.23 ± 0.00 | 0.04 ± 0.00 | 0.05 ± 0.01 | 1.33 ± 0.01 |
Our preliminary estimate indicates that several nanograms of deposited PbTx in a certain region of the human respiratory tract was associated with observed symptoms in upper airway and/or lower airway systems. From the 2000 study in Corpus Christi (8) we observed that a dose rate of 4–5 ng/h in the ET region was associated with observed upper airway symptoms (throat irritation, nasal irritation, and itchy skin), whereas the estimated dose rate in the lung was 0.15–0.2 ng/h, which was not sufficient to cause lower respiratory symptoms. For the study conducted in March 2003, we estimated that the deposited dose in the lower airways (TB and P) for an average 1-h exposure to 37 ± 17 ng/m3 of PbTx air concentration was 3.3 ± 1.5 ng. This dose level in the lower airways seemed to be associated with lower airway symptoms, including cough, wheezing, and chest tightness in asthmatic subjects for the exposure periods. Our data show that exposure to air concentrations as low as 3–5 ng/m3 may be sufficient to relate to upper airway symptoms, and higher PbTx concentrations of 30–40 ng/m3 may be required to cause lower airway symptoms for asthmatics.
Acknowledgments
This research is supported by the National Institute of Environmental Health Sciences (NIEHS) program project P01-ES10594. The authors would like to thank Drs. Lora Fleming, Barbara Kirkpatrick, and Lori Backer for coordinating the field studies.
Literature Cited
- 1.Baden DG. Marine food-borne dinoflagellate toxins. Int Rev Cytol. 1983;82:99–150. doi: 10.1016/s0074-7696(08)60824-4. [DOI] [PubMed] [Google Scholar]
- 2.Forrester DJ, Gaskin JM, White FH, Thompson NP, Quick JA, Henderson GE, Woodard JC, Robertson WD. An epizootic of waterfowl associated with a red tide episode in Florida. J Wildl Dis. 1977;13:160–167. doi: 10.7589/0090-3558-13.2.160. [DOI] [PubMed] [Google Scholar]
- 3.Bossart GD, Baden DG, Ewing RY, Roberts B, Wright SD. Brevetoxicosis in manatees (Trichechus manatus latirostris) from 1996 epizootic: Gross, histologic, and immunohistochemical features. Toxicol Pathol. 1998;26:276–282. doi: 10.1177/019262339802600214. [DOI] [PubMed] [Google Scholar]
- 4.Kirkpatrick B, Fleming LE, Squicciarini D, Backer LC, Clark R, Abraham W, Benson JM, Cheng YS, Johnson D, Pierce RH, Zaias J, Bossart GD, Baden DG. Literature Review of Red Tide: Implication for human health effects. Harmful Algae. 2004;3:99–115. doi: 10.1016/j.hal.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierce RH, Henry MS, Proffitt S, Hasbrouck PA. Red tide toxin (brevetoxin) enrichment in marine aerosol. In: Graneli E, Sundstorm B, Edler L, Anderson DM, editors. Toxic Marine Phytoplankton. Elsevier Science; New York: 1989. pp. 397–402. [Google Scholar]
- 6.Backer LC, Fleming LE, Rowan A, Cheng YS, Benson JM, Pierce RH, Zaias J, Bean J, Bossart GD, Johnson D, Quimbo R, Baden DG. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae. 2003;2:19–28. [Google Scholar]
- 7.Pierce RH, Henry MS, Blum PC, Lyons J, Cheng YS, Yazzie D, Zhou Y. Brevetoxin concentrations in marine aerosol: Human exposure levels during a Karenia brevis harmful algal bloom. Bull Environ Contam. 2003;70:161–165. doi: 10.1007/s00128-002-0170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng YS, Villareal TA, Zhou Y, Gao J, Pierce RH, Wetzel D, Naar J, Baden DG. Characterization of red tide aerosol on the Texas coast. Harmful Algae. 2005;4(1):87–94. doi: 10.1016/j.hal.2003.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baden DG, Mende TJ, Walling J, Schultz DR. Specific antibodies directed against toxins of Ptychodiscus brevis (Florida's red tide dinoflagellate) Toxicon. 1984;22:783–789. doi: 10.1016/0041-0101(84)90161-2. [DOI] [PubMed] [Google Scholar]
- 10.Trainer VL, Baden DG. An enzyme immunoassay for the detection of Florida red tide brevetoxins. Toxicon. 1991;29:1387–1394. doi: 10.1016/0041-0101(91)90126-c. [DOI] [PubMed] [Google Scholar]
- 11.Naar J, Bourdelais A, Tomas C, Kubanek J, Whitney P, Flewelling L, Steidinger KA, Lancaster J, Baden DG. A competitive ELISA to detect brevetoxins from Karenia brevis (formerly Gymnodinium breve) in seawater, shellfish, and mammalian body fluid. Environ Health Perspect. 2002;110:179–185. doi: 10.1289/ehp.02110179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baden DG. Brevetoxins: Unique polyether dinoflagellate toxins. FASEB. 1989;3:1807–1817. doi: 10.1096/fasebj.3.7.2565840. [DOI] [PubMed] [Google Scholar]
- 13.Gawley RE, Rein KS, Kinoshita M, Baden DG. Binding of brevetoxins and ciguatoxin to the voltage-sensitive sodium channel and conformational analysis of brevetoxin B. Toxicon. 1992;30:780–785. doi: 10.1016/0041-0101(92)90014-v. [DOI] [PubMed] [Google Scholar]
- 14.Roszell LE, Schulman LS, Baden DG. Toxin profile are dependent on growth stages in cultured Ptychodiscus brevis. In: Graneli E, Sundstorm B, Edler L, Anderson DM, editors. Toxic Marine Phytoplankton. Elsevier Science; New York: 1989. pp. 403–406. [Google Scholar]
- 15.Baden DG, Tomas CR. Variation in major toxin composition for six clones of Ptychodiscus brevis. Toxicon. 1988;26:961–963. doi: 10.1016/0041-0101(88)90261-9. [DOI] [PubMed] [Google Scholar]
- 16.Pierce RH, Brown RC, Kucklick JR. Analysis of Ptychodiscus brevis toxins by reverse phase HPLC. In: Anderson DM, White AW, Baden DG, editors. Toxic Dinoflagellates. Elsevier Science; New York: 1985. pp. 309–314. [Google Scholar]
- 17.Hua Y, Lu W, Pierce RH, Cole RP. On-line high performance liquid chromatography-electrospray ionization mass spectrometry for the determination of brevetoxins in red tide algae. Anal Chem. 1995;67:1815–1823. doi: 10.1021/ac00107a010. [DOI] [PubMed] [Google Scholar]
- 18.Winker A, Siebers D, Leweck K. Determination of sodium in seawater by use of ion-selective electrodes. GIT Fachz Lab. 1982;26:228–229. [Google Scholar]
- 19.ICRP. Human Respiratory Tract Model for Radiological Protection, Publication 66. Ann ICRP. 1994;24:1–3. [PubMed] [Google Scholar]
- 20.Bourdelais AJ, Campbell S, Jacocks H, Naar J, Wright JLC, Carsi J, Baden DG. Brevenal is a natural inhibitor of brevetoxins action in sodium channel receptor binding assays. Cell Molecular Neurobiol. 2004;24:553–563. doi: 10.1023/B:CEMN.0000023629.81595.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzgerald JW. Marine aerosols: A review. Atmos Environ. 1991;25A:533–545. [Google Scholar]
- 22.Backer LC, Kirkpatrick B, Fleming LE, Cheng YS, Pierce RH, Bean J, Clark R, Johnson D, Wanner A, Tamer R, Baden DG. Occupational exposure to aerosolized brevetoxins during Florida red tide events: Impacts on a healthy worker population. Environ Health Perspect. doi: 10.1289/ehp.7502. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


