Figure 8. Schematic representation of nuclear-mitochondrial communication response to OXPHOS defect in V425 colorectal cancer cells.
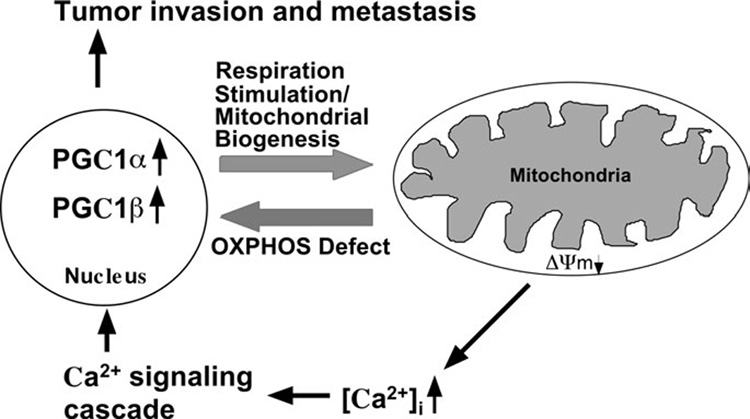
V425 colorectal cancer cells harbor a severe defect in COX activity and a partial defect in mitochondrial respiration, membrane potential (ΔΨm) and cytoslic calcium [Ca2+]c buffering ability. Partial defect in ΔΨm and [Ca2+]c buffering ability can stimulate the Ca2+ signaling cascade which in turn might activate genes involved in tumor invasion and metastasis. The defect in the activity of complex IV (and possibly also complex I) signals the nucleus to activate the expression of PGC-1 family of transcriptional coactivators that regulate OXPHOS gene expression. Increased expression of PGC-1α and PGC-1β coactivators in turn stimulates respiration by increasing mitochondrial biogenesis, which in V425 appears to involve direct coactivation of a broad but specific set of OXPHOS genes (such as SDH, COX IV, Cyt c, VDAC1 and Bcl-xL). This paradigm provides a link between deleterious mtDNA mutations, partial OXPHOS defects and cancer progression.
