Figure 8.
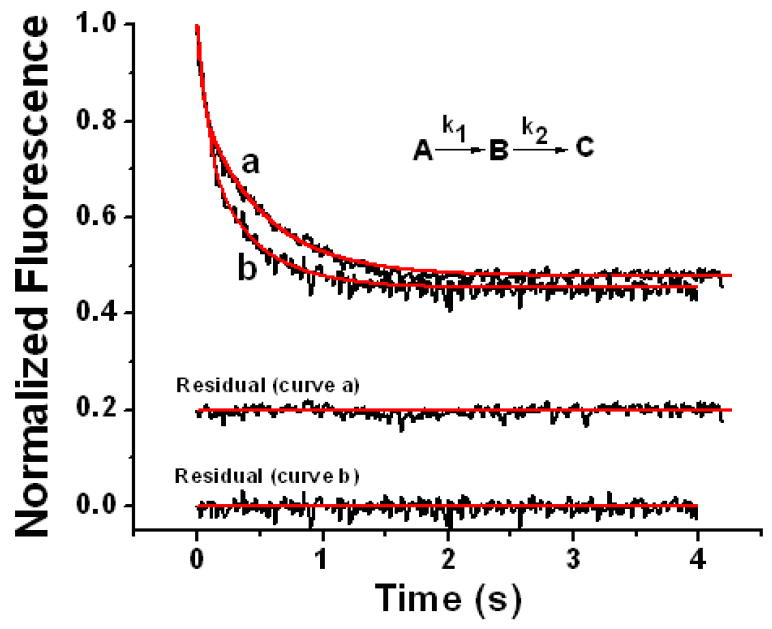
Stopped-flow fluorescence quenching by O2 oxidation of Fe2+ prebound to variants #1 (curve a) and #2 (curve b) containing 48 Fe2+/shell. Fitted curves are shown in red. The rate constants and intrinsic fluorescence constants for variant #1 from curve-fitting (curve a) are: k1 = 19.0 ± 3.1 s-1 and k2 = 1.86 ± 0.04 s-1, IA = 1.01 ± 0.02 μM-1 on a per iron dimer basis, IB = 0.655 ± 0.021 μM-1 dimer, and IC = 0.0425 + 0.0012 μM-1 dimer (curve a); and for variant #2 (curve b) are: k1 = 16.6 ± 2.3 s-1 and k2 = 2.38 ± 0.15 s-1, IA = 0.711 ± 0.011 μM-1 dimer, IB = 0.320 ± 0.030 μM-1 dimer, and IC = 0.0158 ± 0.0013 μM-1 dimer (curve b) from eq 5 with the equations for the concentrations of species given elsewhere.31 The residual is the difference between the fitted and experimental curves. The stated rate constants and associated errors are averages and standard deviations, respectively, from curve fits to three separate kinetic runs. The observed quenching kinetics is from the oxidation of the prebound Fe2+ and not from the binding of Fe2+ itself. Final conditions: anaerobic 1.5 μM apo-variants containing 48 Fe2+/shell in 50 mM Mops pH 7.15 rapidly mixed with H2O saturated with 100% O2, 25 °C.
