FIGURE 4. Disruption of lipid rafts inhibits TNF-α-induced RhoA activation.
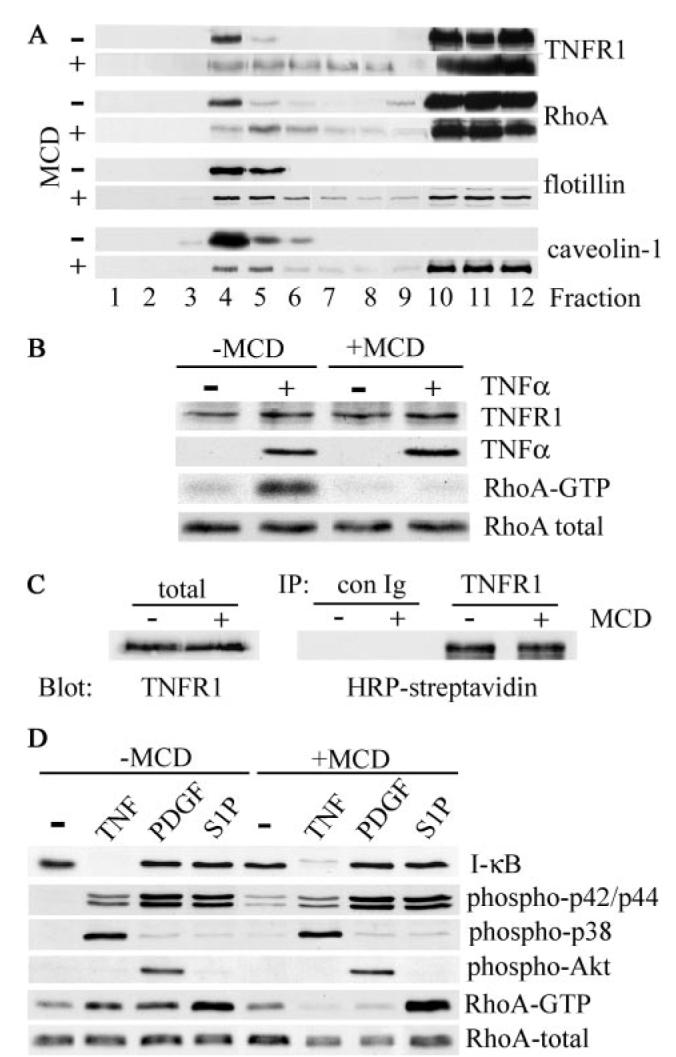
A, human airway smooth muscle cells were treated with (+) or without (−) 10 mm MCD for 1 h at 37 °C prior to extraction with cold Triton X-100 and sucrose density gradient fractionation. The distribution of TNFR1, RhoA, and the raft-resident proteins flotillin-1 and caveolin-1 was determined by immunoblotting. B, cells, treated with (+) or without (−) 10 mm MCD for 30 min at 37 °C, were stimulated with TNF-α (200 ng/ml) for 5 min at 37 °C, extracted, and analyzed by immunoblotting for TNFR1 and RhoA expression and TNF-α binding. Activated RhoA (RhoA-GTP) was isolated using a glutathione S-transferase fusion protein containing the Rho binding domain of Rhotekin as described under “Experimental Procedures.” C, human airway smooth muscle cells were surface-biotinylated with sulfo-NHS-biotin prior to treatment with (+) or without (−) MCD as in B. Cell extracts were immunoprecipitated (IP) with control (con Ig) or anti-TNFR1 antibody, and cell surface-associated TNFR1 was detected by blotting with HRP-streptavidin. To control for protein input, total cell extracts (total) were analyzed by immunoblotting for expression of TNFR1. D, cells treated with (+) or without (−) MCD as in B were stimulated for 5 min at 37 °C with TNF-α (200 ng/ml), PDGFBB (50 ng/ml), or S1P (1 μm). Cell extracts were separated by SDS-PAGE and analyzed by immunoblotting with antibodies against the indicated proteins. RhoA-GTP was isolated as described in B.
