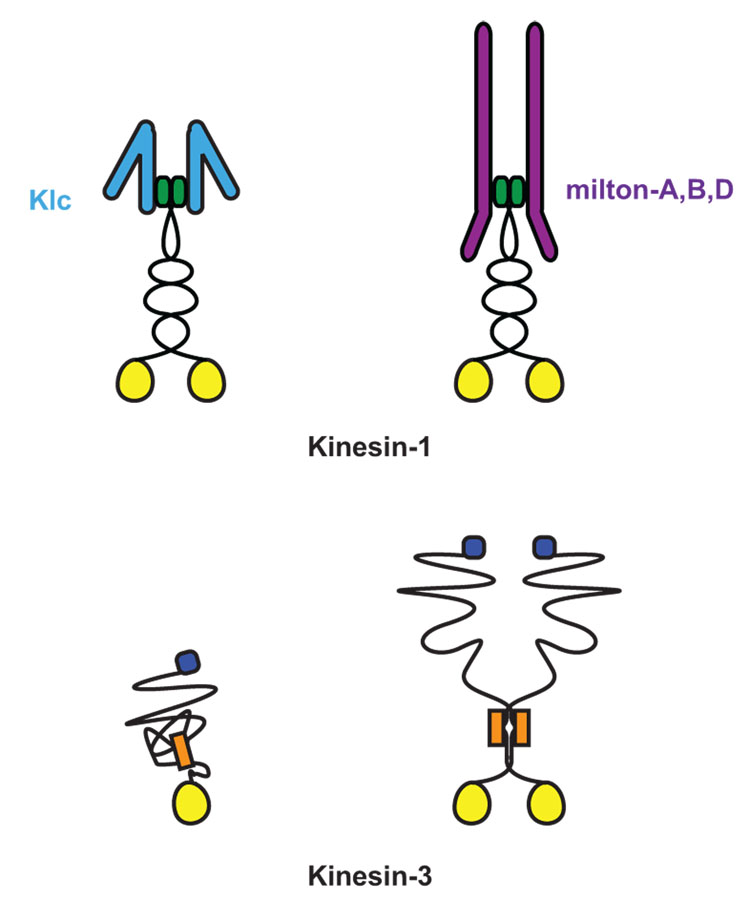
Figure 1. Meet the family of Kinesin-1 and Kinesin-3.
Kinesins are defined by their highly conserved ATP binding and microtubule binding motor domain (yellow circle) [8]. Both Kinesin-1 and Kinesin-3 family members have their motor domain at their N-termini and move toward the (+)-end microtubules. The Kinesin-1 family subfamily of KIF5, or kinesin heavy chain (KHC), is a homodimer that dimerizes via coiled-coil domains at its neck. KHC associates with two kinesin light chains (KLC) to link it to multiple cargo complexes [7]. Although initially believed to act as an obligate tetramer with KLC, KHC can also associate with cargos via a specialized adaptors independent of KLC, as is the case with the mitochondrial adaptor protein, Milton [•36]. In contrast, Kinesin-3 family members have been found as both monomers and dimers and are able to associate to vesicular cargo directly. Kinesin-3 family members share a conserved fork-head association domain (orange box) and multiple coiled-coil domains at the neck of the motor [7,8]. The defining motor of the Kinesin-3 family, Unc-104, has a pleckstrin homology domain (blue square) that is necessary for its association with synaptic vesicle precursors [24].