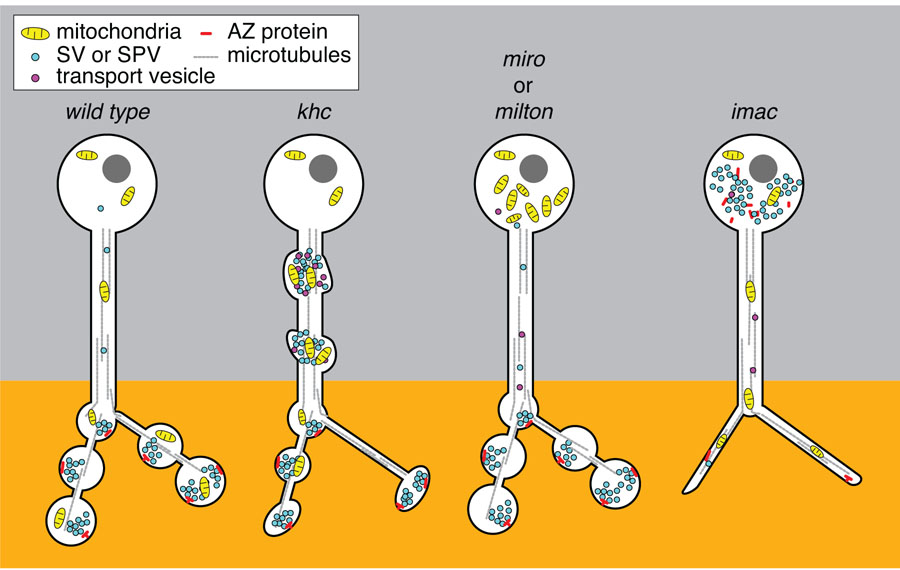
Figure 2. Distinct functions of Kinesin-1 and Kinesin-3 family motors for transport in the axon.
The presynaptic compartment of the neuron is normally enriched with mitochondria and SVs in close apposition to a density of active zone proteins. Loss of the lone Kinesin-1 motor in the fly, KHC, leads to accumulations of mitochondria, SPV as well as post-golgi transport vesicles in the axon [7]. This is striking when compared to what occurs in the absence of either milton or Miro, the adaptor complex that associates KHC to mitochondria. Mitochondria are stranded in the cell body of both milton and miro mutants and do not generate traffic jams in the axonal processes or disrupt the distribution of SVs or active zone proteins [•36,38]. One interpretation of this discrepancy between the phenotype of Milton/Miro and KHC is that the traffic jams in the khc mutants are due to the mislocalization of additional cargos of KHC. For example, through binding of the JIP1 signaling complex associated with KLC and transport vesicles, decreased KHC dependent transport could compromise both the integrity of the cytoskeletal network as well as the signal for the proper sorting of cargos. In contrast, loss of the Kinesin-3 family motors Unc-104 or its fly homologue, Imac, leads to a severe reduction of synaptic vesicles (SVs) at terminals and an increase of SVs stranded in the cell body [7]. Mitochondrial distribution and axon guidance and growth remains normal in unc-104 and imac mutants demonstrating their specialized role for SVP transport. In addition, in the absence of Imac, morphologically mature synaptic endings do not form and active zone proteins are stranded in the cell body along with a notable reduction of active zones (AZ) at the synapse [••9]. Although loss of KHC does disrupt the normal trafficking of SVs and demonstrates a reduction of synapses, the severity of the imac phenotype implies that it is likely the primary motor to initiate the transport of SVs and as yet unidentified proteins critical for morphological synapse development.