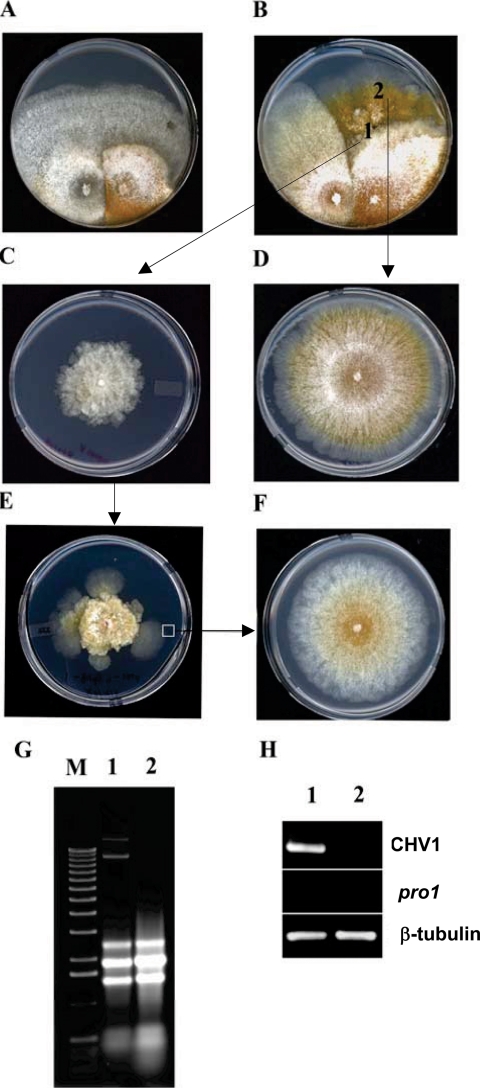
FIG. 7.
Requirement of the pro1 gene for CHV1-EP713 virus maintenance. (A and B) Anastomosis-mediated transfer of hypovirus CHV1-EP713 from strain EP713 (left side of each panel) to wild-type strain EP155 (A) and pro1 disruption mutant Δpro1T6 (B). (C) Subcultures taken from the converted, CHV1-EP713-infected pro1 disruption mutant culture at a point near the initial contact between the paired colonies (position 1) exhibited virus infection symptoms that included loss of orange pigment production and altered colony morphology (Fig. 4D). (D) Subcultures taken from rapidly growing sectors originating from the converted pro1 disruption mutant (position 2) exhibited a virus-free colony phenotype. (E) With successive subculturing (transfer every 7 days), the CHV1-EP713-infected Δpro1T6 mycelia produced rapidly growing sectors at a high frequency and the colonies derived from those sectors always exhibited a virus-free phenotype (F). (G) Total RNA was extracted from colonies originating from mycelia taken from positions 1 and 2 (C and D, respectively) and analyzed on an agarose gel. The slowly migrating bands present in lane 1 but absent in lane 2 are full-length and defective CHV1-EP713 replicative double-stranded RNAs. (H) RT-PCR analysis detected a virus-specific product (lane 1) from colonies originating from position 1 (C) but not from position 2 (D). Primers specific for CHV1-EP713 genome RNA and pro1 mRNA were used along with primers specific for β-tubulin mRNA as reaction and loading controls.