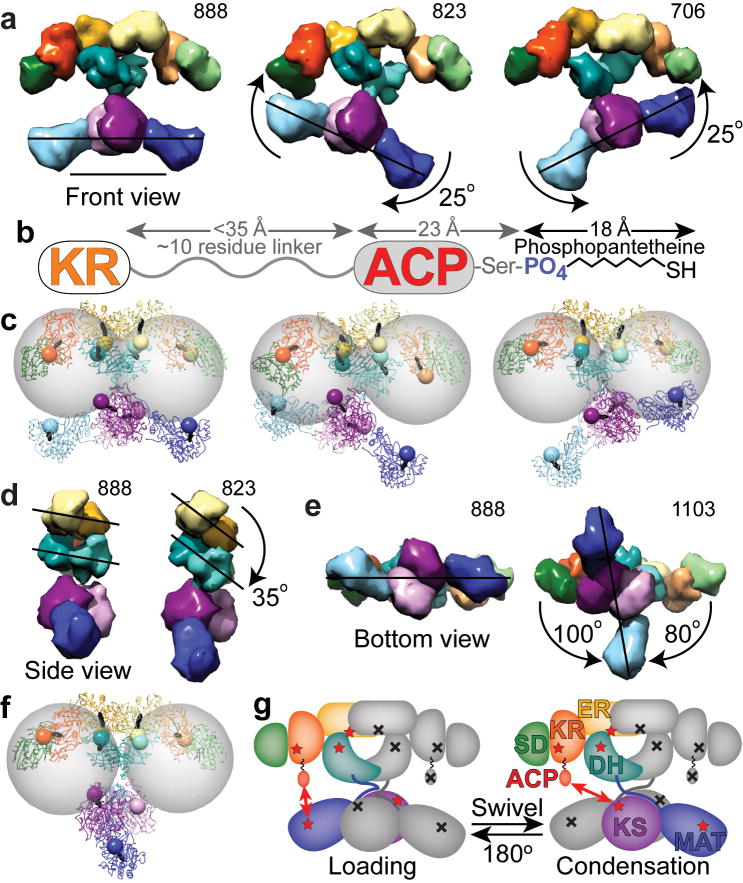
Figure 5.
Changes in domain position bring catalytic domains into proximity of the ACP to facilitate catalytic interactions. (a) Lower portion swings relative to upper portion. (b) Based on crystal and NMR structures of the human and rat ACP domains36,40, and from analysis of sequence conservation among metazoan FAS, the KR-ACP linker likely consists of approximately 10 residues between Lys2109 through Arg2120 (rat FAS numbering). When fully extended this linker could be up to 35 Å in length. The ACP is approximately 23 Å long from its N-terminus to the phosphopantetheinylated Ser2151. The phosphopanthetheine must extend approximately 18 Å from the ACP to reach the active site within each catalytic domain. (c) After roughly modeling a rigid, extended phosphopantetheine into each active site pocket, the phosphate was rendered as an 8 Å radius sphere. Gray spheres of 55 Å radius indicate the distance that the ACP domains could reach from a fixed tether point at the C-terminus of the KR. (d) Side view of FAS with KR and SD removed from one subunit revealing rotation of the DH and ER domains. (e,f) Lower portion swivels relative to upper portion. (gi) Full 180° swiveling of the lower portion of the structure occurs during each catalytic cycle to explain the FAS activity of a heterodimer composed of a wild-type subunit (colored domains with red stars) partnered with a mutant subunit lacking all 7 functionalities (indicated by gray domains with black crosses). Domains of FAS are colored as in Figure 2. Scale bar in a represents 100 Å.