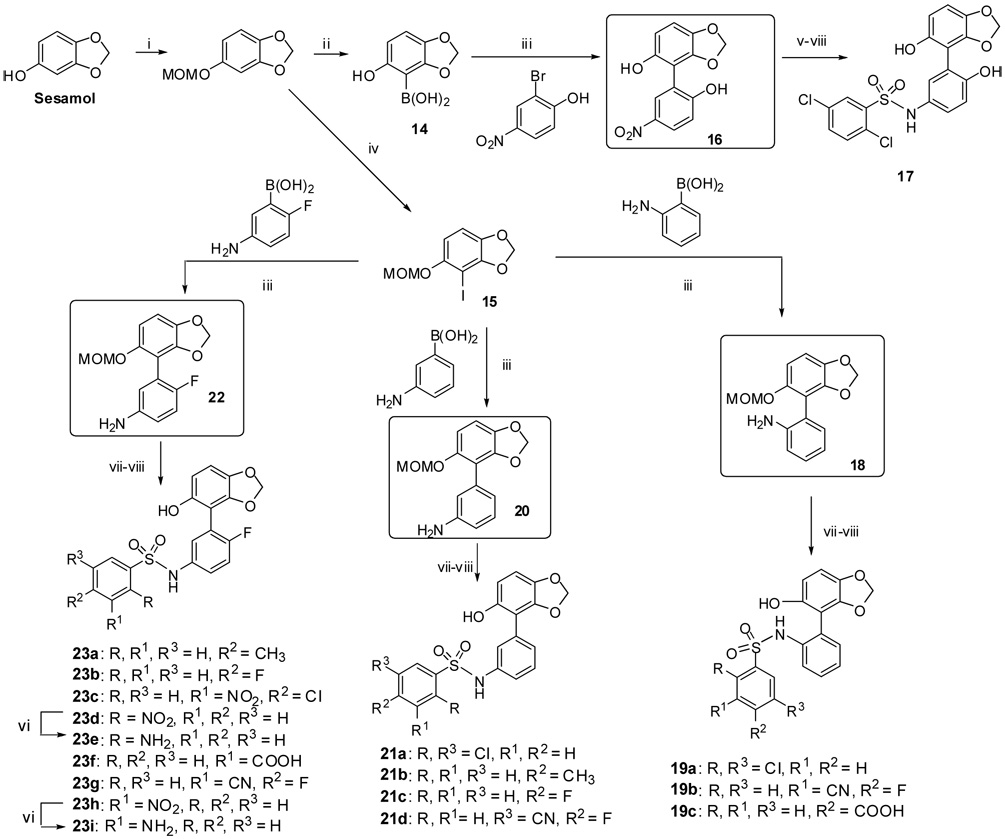
Scheme 2.
Reagents and conditions: i. NaH, MOMCl, DMF, 0 °C-RT, 98%, ii. n-BuLi, THF, B(OMe)3, −78 °C, then 10% HCl, RT tech-grade (50%). iii. 15 (1 eq, 2 mmol), boronic acids (1.8–2 eq), Pd (PPh3)4 (5–10 mol%) , K2CO3,(3–3.3 eq), DME:EtOH:H2O (3:2:1 mL), Microwave initiator, 15 min., 160 °C, 90–95% (except for the 16 which only obtained in 20% yield based on recovered SM), iv. n-BuLi, THF, I2, 0 °C-RT v. Boc2O, DMAP, THF, Reflux, 3–4 h, 95%, vi. H2, 10%Pd-C, MeOH:EtOAc (6:2 mL), 12 Psi, 1 h, 80–85%, vii. ArSO2Cl, Py, 100% viii. 2M HCl,THF:MeOH, 90%.