Fig. 1.
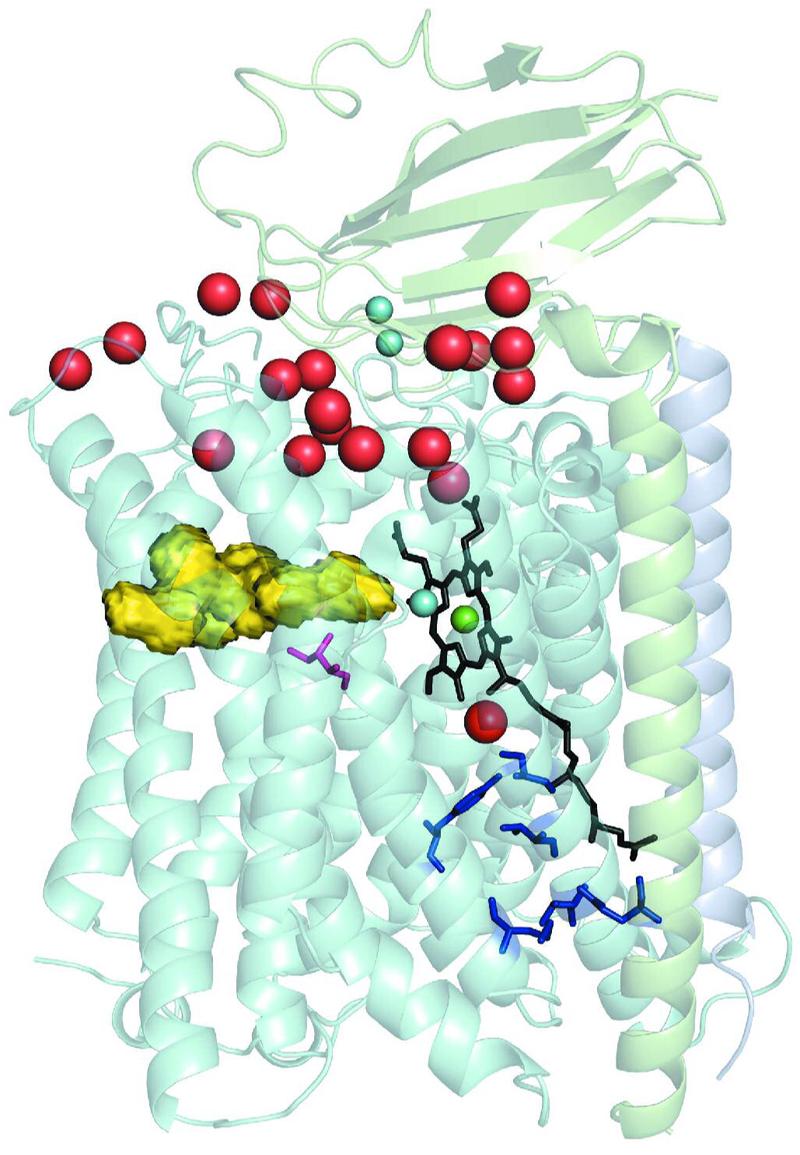
Representation of the Thermus cytochrome ba3 structure using translucent ribbons. The molecule is embedded in a phospholipid bilayer whose OUTside is roughly parallel to a plane through the lowest water molecules (red spheres) and whose INside lies just below the blue spheres near the bottom of the molecule. Subunit II, shown in light gray, is at the top with its single transmembrane element running down the right side of the structure. Subunit IIa, being a very short transmembrane segment running from N-(top) to its C-terminal (bottom), is shown in light mauve. Subunit I, shown in light blue, is composed of thirteen transmembrane segments. Within the helices of subunit I are two heme moeities: The a3-heme is shown in black with its long, hydrophobic, geranylgeranyl tail running from near the center of the molecule downward, the Fea3 at the center of the a3-heme is shown in green, and just to the left is the cyan CuB. To reduce clutter in the picture the b-heme, that lies behind the a3-heme is not shown. The dinuclear CuA center is shown as a pair of small cyan spheres near top and center. The larger red spheres at the top of the figure represent some of the water molecules residing near or in the space between the globular domain of subunit II and the concavity of subunit I into which the globular domain of subunit II intrudes. The yellow object to the left just above center represents the inner surface of the oxygen uptake channel that leads from near the center of the membrane bilayer to the dnc19. The pink colored residue center left and below the oxygen channel represents residue I-Ile235, which is replaced by a glutamate residue in A1-type oxidases. The blue structures near the bottom right of the structure represent the currently identified elements of the K-pathway for proton uptake with the lowest representing II-Glu15 whose Cα atom is situated near the N-terminus of subunit II; other residues shown are listed in the text. The red ball just below the green Fea3-atom corresponds to HOH O32 and might be thought of as the exit terminus of the K-path.
